For US Healthcare Professionals
I am a:
IN MODERATE TO SEVERE PLAQUE PsO
Established safety profile through 5 years
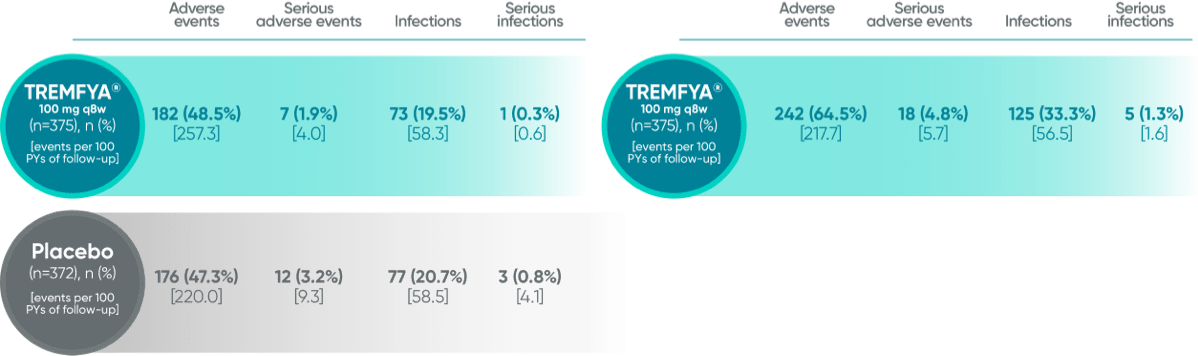
Adverse events in the 16-week, placebo-controlled period of the VOYAGE 1 and VOYAGE 2 pooled clinical trials1,2


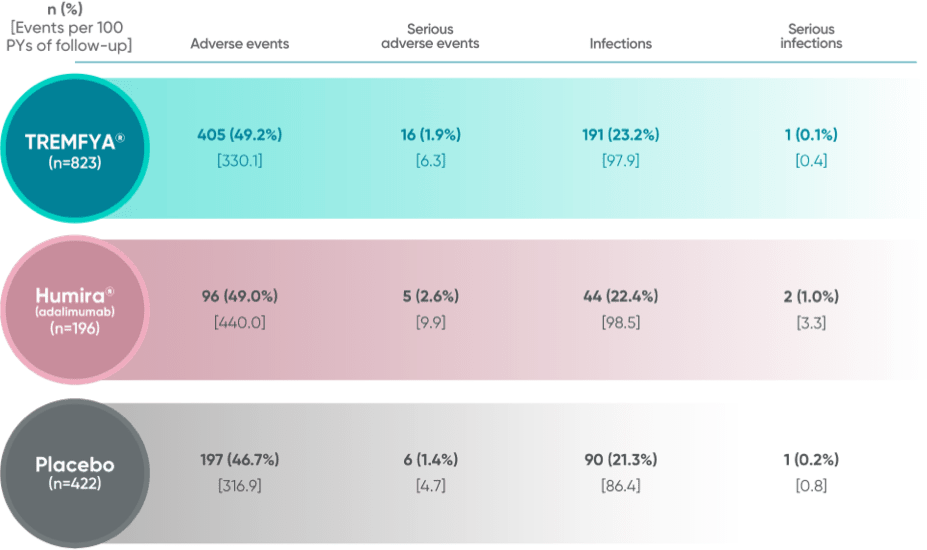

The most common (≥1%) infections were upper respiratory infections, gastroenteritis, tinea infections, and herpes simplex infections; all cases were mild to moderate in severity and did not lead to discontinuation of TREMFYA®
Pooled safety data from VOYAGE 1 and VOYAGE 2 through 5 years (Week 264)2*


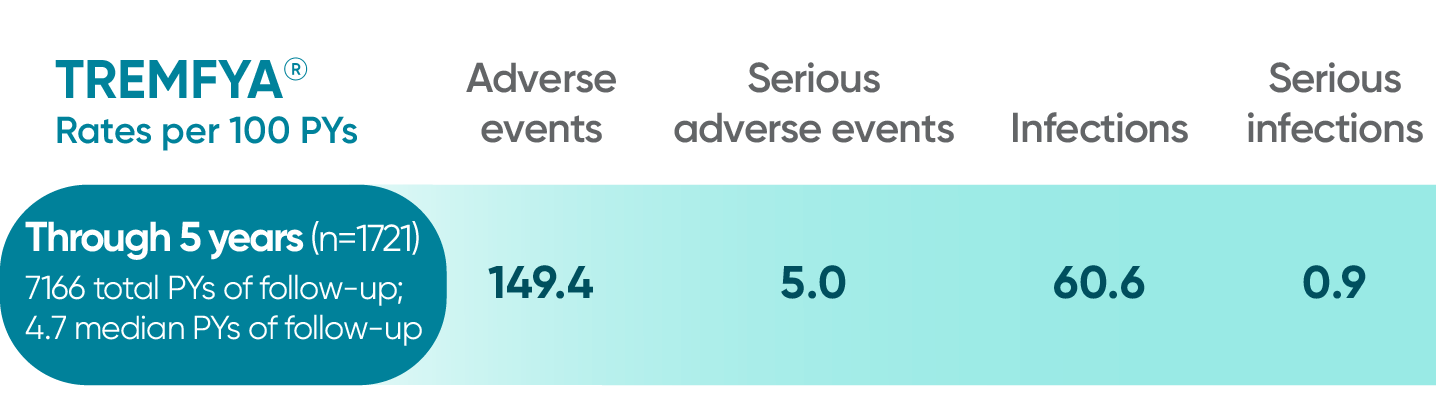

*Safety summary includes all patients exposed to TREMFYA®.
Humira is a registered trademark of Abbvie Biotechnology Ltd. Corporation.
PYs=patient-years.
References: 1. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Data on file. Janssen Biotech, Inc.
IN ACTIVE PsA
Established safety profile through 2 years*
Combined safety across DISCOVER 1 and DISCOVER 2 through Week 24 and Year 11†
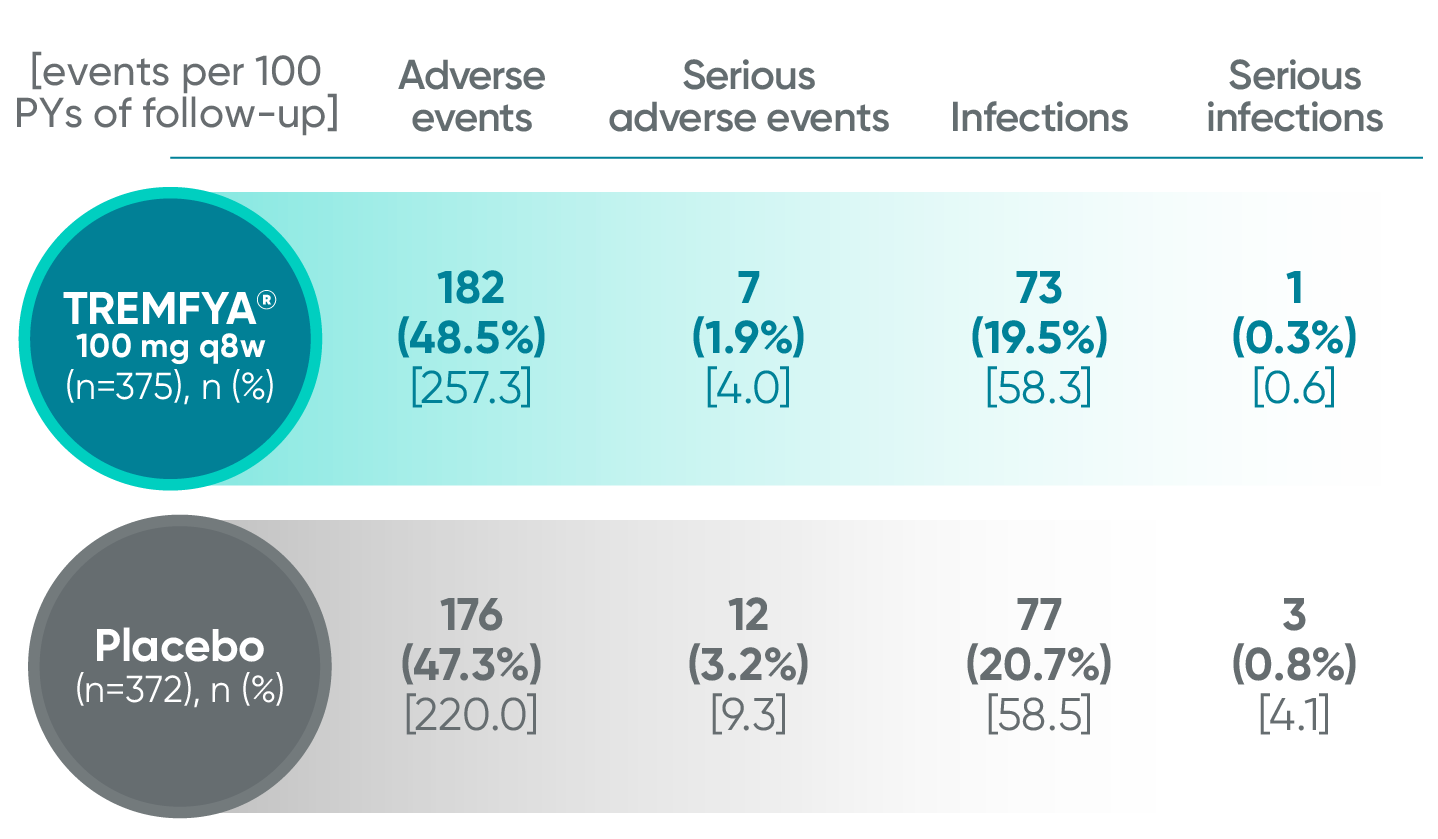
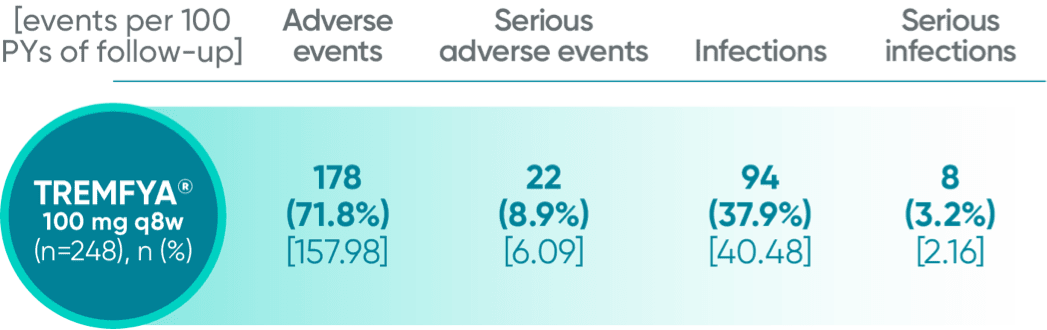
Adverse events reported in the placebo-controlled phase through Week 24 combined across DISCOVER 1 and DISCOVER 2
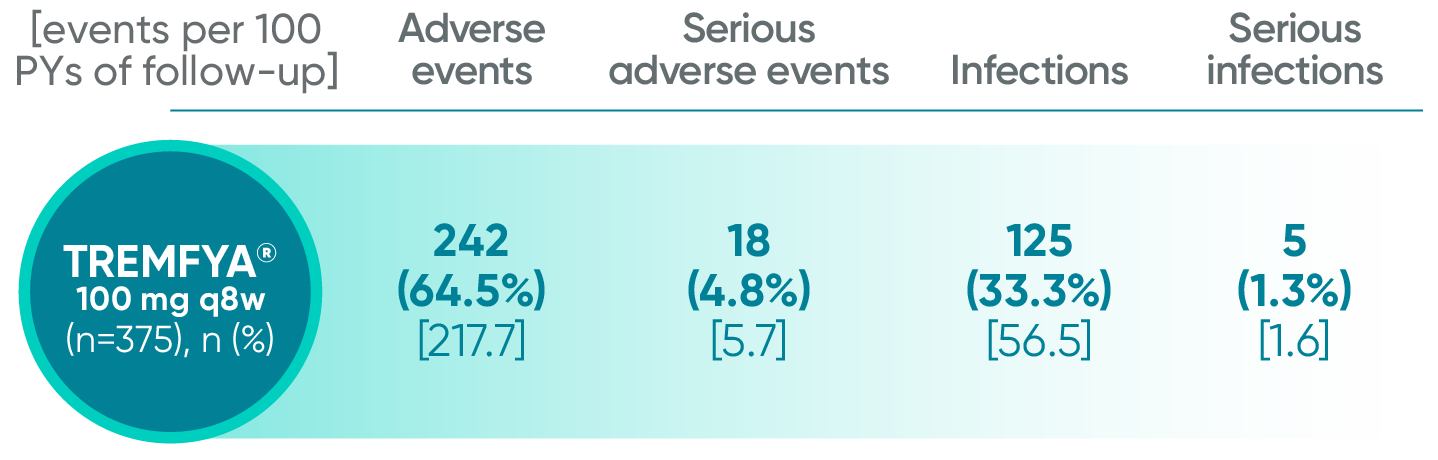
Adverse events reported through Year 1‡ combined across DISCOVER 1 and DISCOVER 2


Adverse events reported through Year 1‡ combined across DISCOVER 1 and DISCOVER 2


In the 24-week, placebo-controlled period of the combined DISCOVER 1 and DISCOVER 2 clinical trials
- The overall safety profile observed in patients with active PsA treated with TREMFYA® is generally consistent with the profile in patients with plaque PsO, with the addition of bronchitis (occurred in 1.6% and 1.1% of patients in the TREMFYA® q8w group and placebo group, respectively) and neutrophil count decreased (occurred in 0.3% and 0% of patients in the TREMFYA® q8w group and placebo group, respectively)2:
- —The majority of events of neutrophil count decreased were mild, transient, not associated with infection, and did not lead to discontinuation
Adverse events reported through end of study (112 weeks) in DISCOVER 21


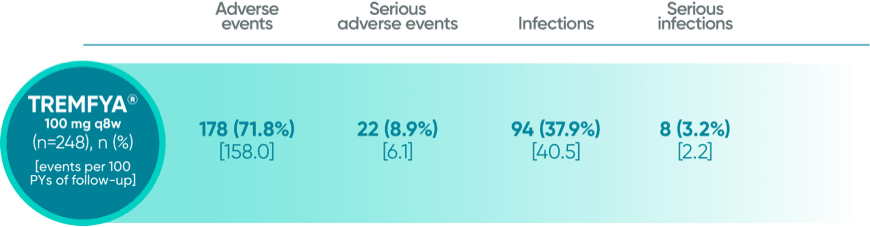
*Through Week 112 in DISCOVER 2.
†1 Year is defined as 60 weeks (through end of study) in DISCOVER 1 and 52 weeks in DISCOVER 2.
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
Extensive patient experience
13
years of
clinical studies*
25
ongoing and completed
clinical studies†
~251K
patient-years
of experience1‡
TREMFYA® is contraindicated in patients with a history of serious hypersensitivity reaction to guselkumab or any of its excipients. Warnings and precautions include infections, tuberculosis (TB), hypersensitivity, and immunizations. Initially evaluate for TB and monitor patients for signs and symptoms of TB infection during and after treatment.
Most common (≥1%) adverse reactions associated with TREMFYA® include upper respiratory infections, headache, injection site reactions, arthralgia, bronchitis, diarrhea, gastroenteritis, tinea infections, and herpes simplex infections.

No labeled warnings or precautions for malignancy, inflammatory bowel disease, or MACE2


No routine lab monitoring required during treatment across current approved indications2§


Established safety profile based on extensive patient experience
*Based on a phase 1 clinical trial that was initiated in June 2009.
†Represents completed and ongoing PsO and PsA clinical studies.
‡Based on worldwide estimated cumulative number of patient-years from launch in July 2017 through June 2022. Patient exposure was estimated by calculation from distribution data.
§As of August 2022.
MACE=major adverse cardiovascular events (cardiovascular death, myocardial infarctions, and cardiovascular events [strokes]).
References: 1. Data on file. Janssen Biotech, Inc. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.


