For US Healthcare Professionals
I am a:
ECLIPSE study design
ECLIPSE: Phase 3, double-blind trial (n=1048)1,2




Cosentyx is a registered trademark of Novartis AG.
Patient eligibility
- ≥18 years of age
- Moderate to severe plaque psoriasis (IGA score ≥3; PASI score ≥12, BSA involvement ≥10%) for at least 6 months
- Candidates for phototherapy and/or systemic treatment
Overall study population1




ECLIPSE statistical methods
This study utilized a step-down approach to control for multiple testing. The first major secondary endpoint did not achieve statistical significance for superiority; therefore, the remaining P values are nominal and not included in the presentation.




Nonresponder imputation (NRI) methods were used for analysis.
*Superiority for the first major secondary endpoint, PASI 75 at both Week 12 and 48, was not achieved (TREMFYA® 84.6% vs Cosentyx® 80.2%; P=0.062); therefore, the remaining P values are nominal and not included in the presentation.
BMI=body mass index; BSA=body surface area; IGA=Investigator's Global Assessment; PASI=Psoriasis Area and Severity Index.
References: 1. Data on file. Janssen Biotech, Inc. 2. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839.
TREMFYA® demonstrated superiority vs Cosentyx®
for PASI 90 response at Week 481,2
ECLIPSE: Primary endpoint at Week 48
†P<0.001 vs Cosentyx®.
VOYAGE pivotal trials' co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
Psoriasis Symptoms and Signs Diary (Week 16): Greater improvements in symptoms of psoriasis (itch, pain, stinging, burning, and skin tightness).3
PASI 75 and IGA 0/1 at Week 12 (secondary endpoints at Week 12)
- PASI 75: TREMFYA® 89% (477/534), Cosentyx® 92% (471/514)
- IGA 0/1: TREMFYA® 86% (457/534), Cosentyx® 86% (444/514)
Due to the results of the step-down approach to control for multiple testing, nominal P values for PASI 75 at Week 12 are not presented and efficacy comparisons cannot be made.
IGA 0/1 at Week 12 was a prespecified exploratory endpoint that was not adjusted for multiplicity; P value was considered nominal.
Results based on ECLIPSE, a single study of TREMFYA® vs Cosentyx®.
Nonresponder imputation (NRI) methods were used for analysis.
There were no new safety findings observed for either TREMFYA® or Cosentyx® in this study.
IGA=Investigator’s Global Assessment, IGA score of cleared (0) or minimal (1) using a 5-point scale of overall disease severity; PASI=Psoriasis Area and Severity Index.
References: 1. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. 2. Data on file. Janssen Biotech, Inc. 3. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
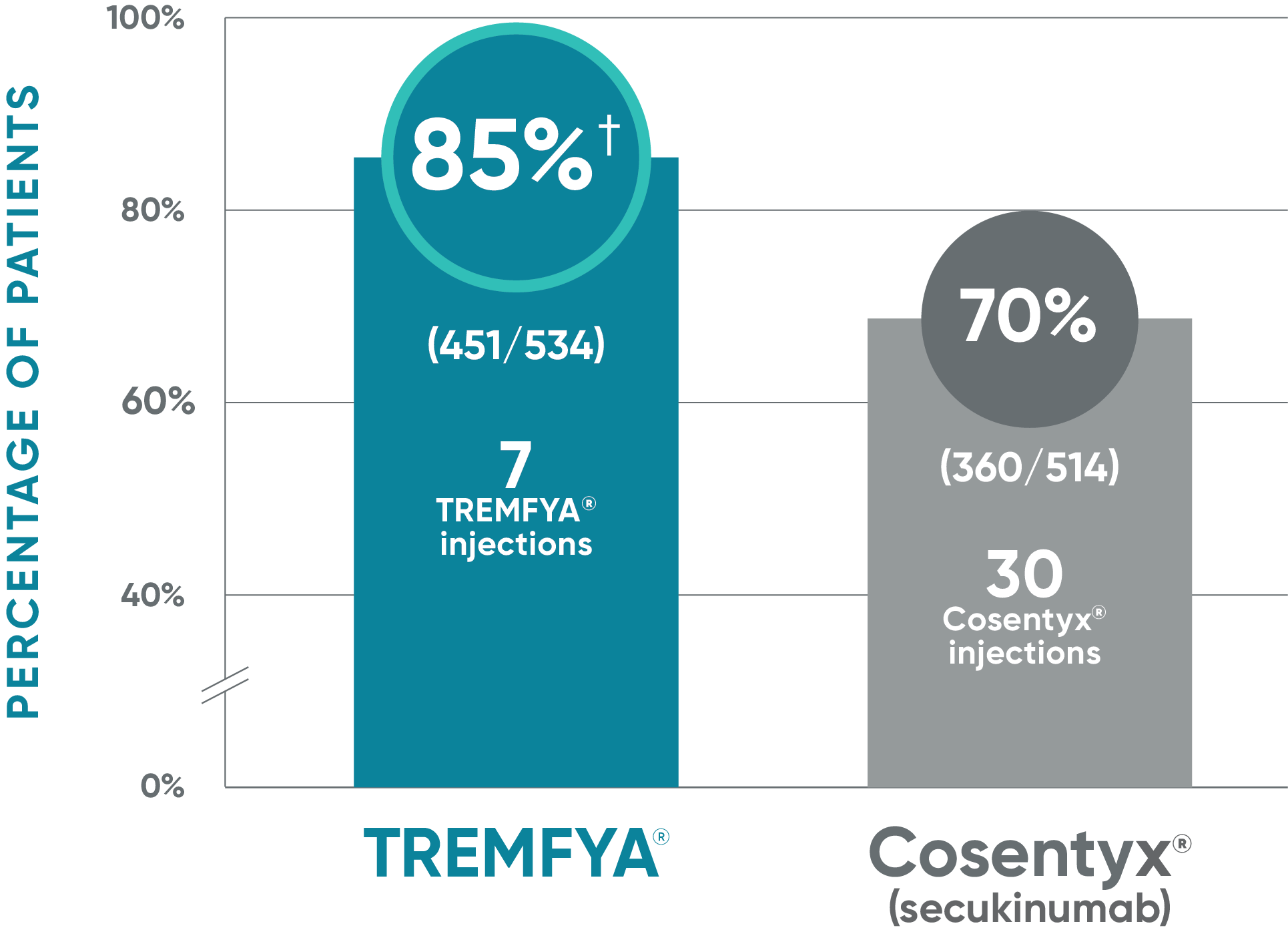
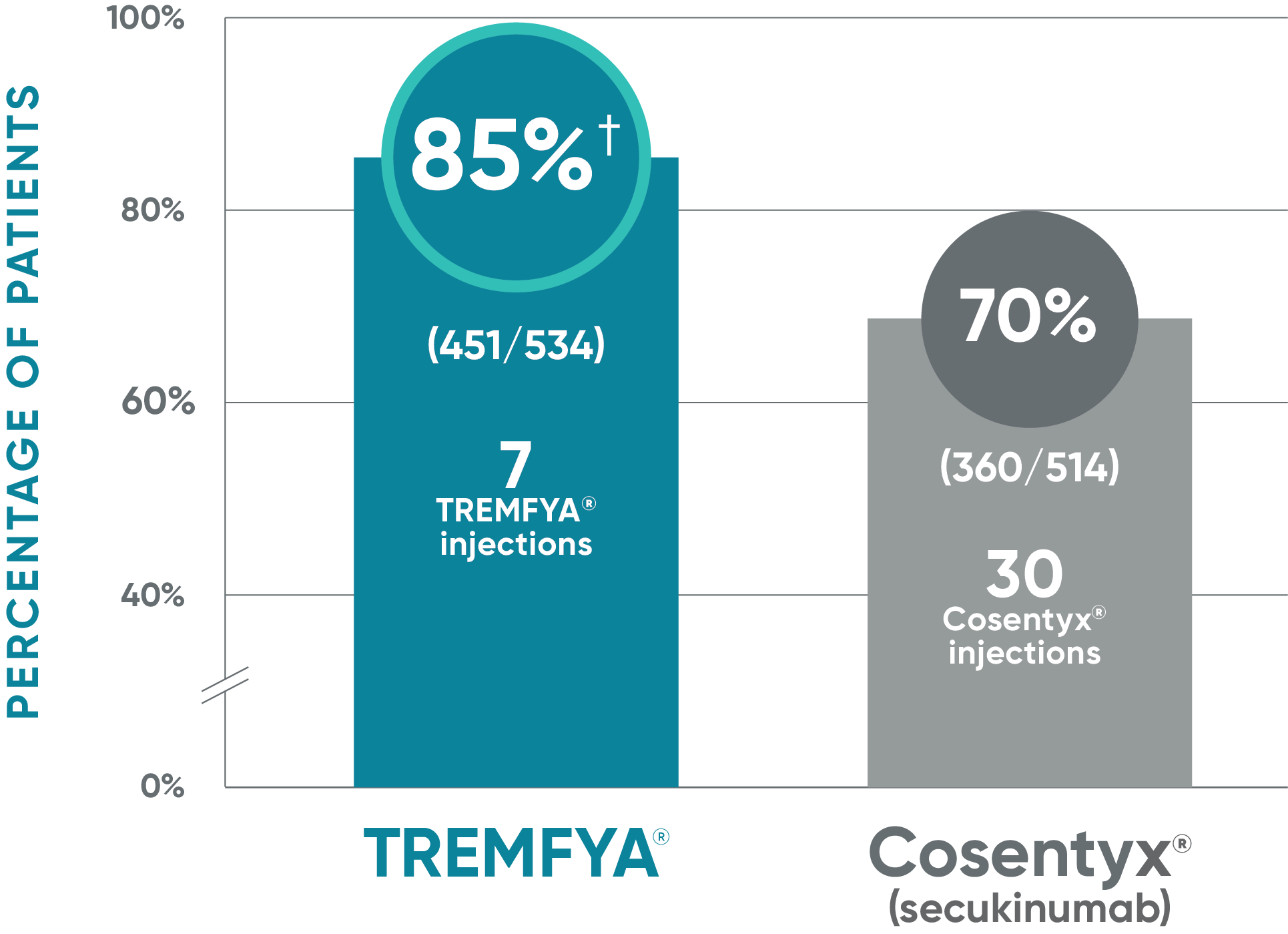
TREMFYA®: IGA 0* at Week 481,2
Due to the results of the step-down approach to control for multiple testing, nominal P values for IGA 0 at Week 48 are not presented and efficacy comparisons cannot be made
ECLIPSE: Major secondary endpoint at Week 48 (NRI)
VOYAGE pivotal trials' co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
Psoriasis Symptoms and Signs Diary (Week 16): Greater improvements in symptoms of psoriasis (itch, pain, stinging, burning, and skin tightness).3
Results based on ECLIPSE: a single study of TREMFYA® vs Cosentyx®.
*IGA 0=proportion of patients who achieved an IGA score of cleared (0) using a 5-point scale where psoriatic lesions are graded by the investigator for induration, erythema, and scaling on a scale of 0 to 4: cleared, except for discoloration (0), minimal (1), mild (2), moderate (3), or severe (4).
References: 1. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. 2. Data on file. Janssen Biotech, Inc. 3. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
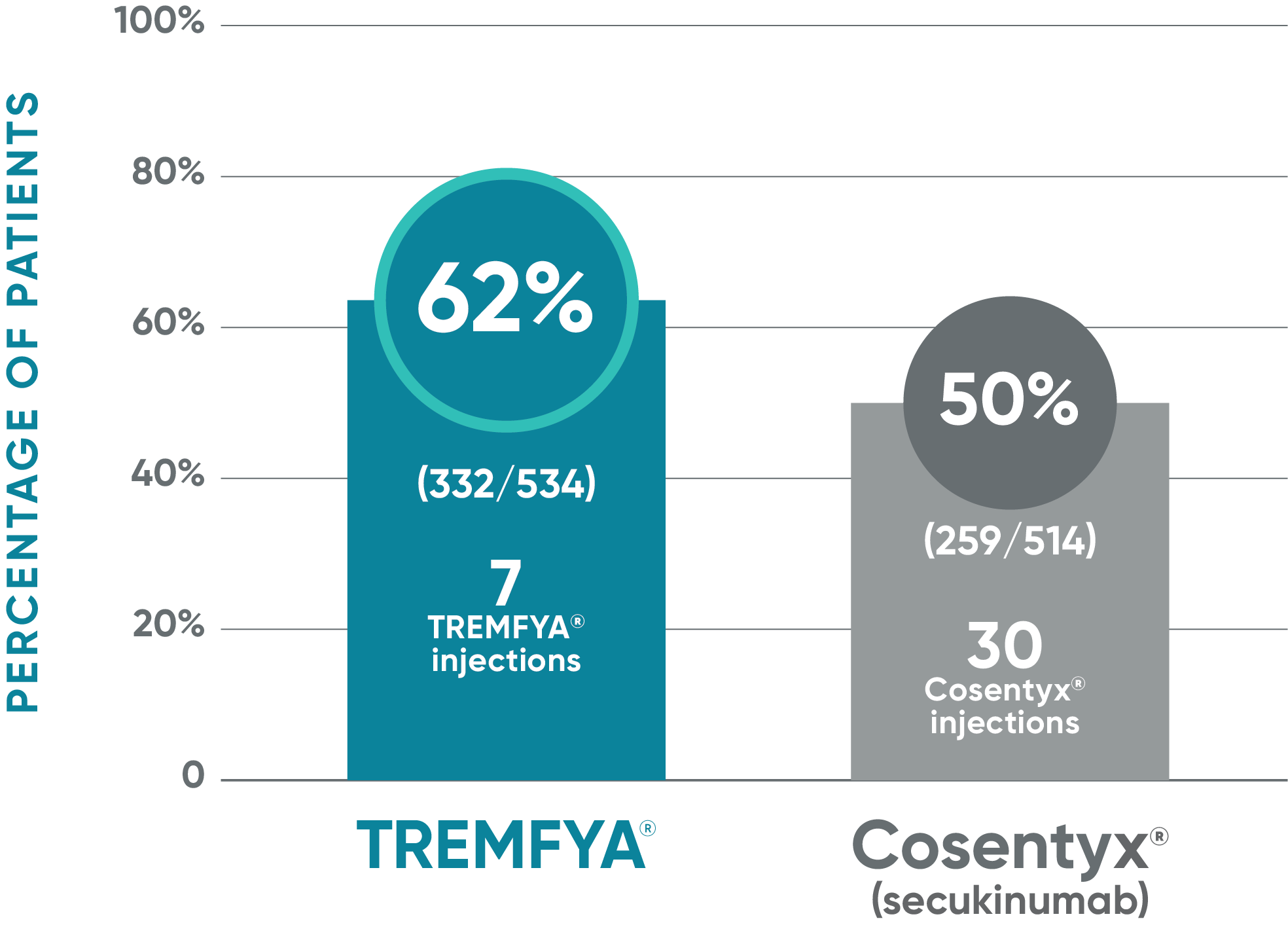
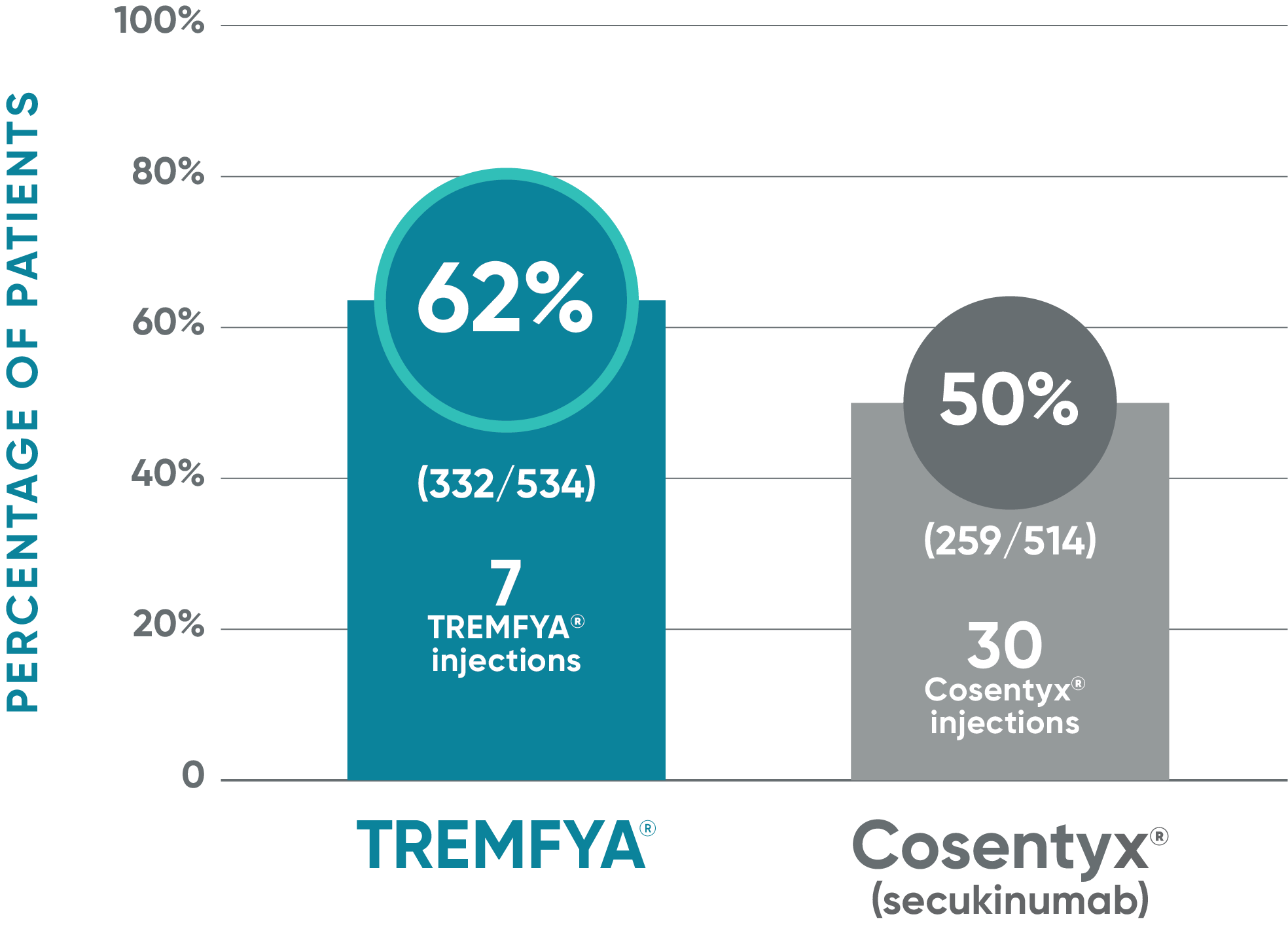
TREMFYA®: PASI 100* at Week 481,2
Due to the results of the step-down approach to control for multiple testing, nominal P values for PASI 100 at Week 48 are not presented and efficacy comparisons cannot be made
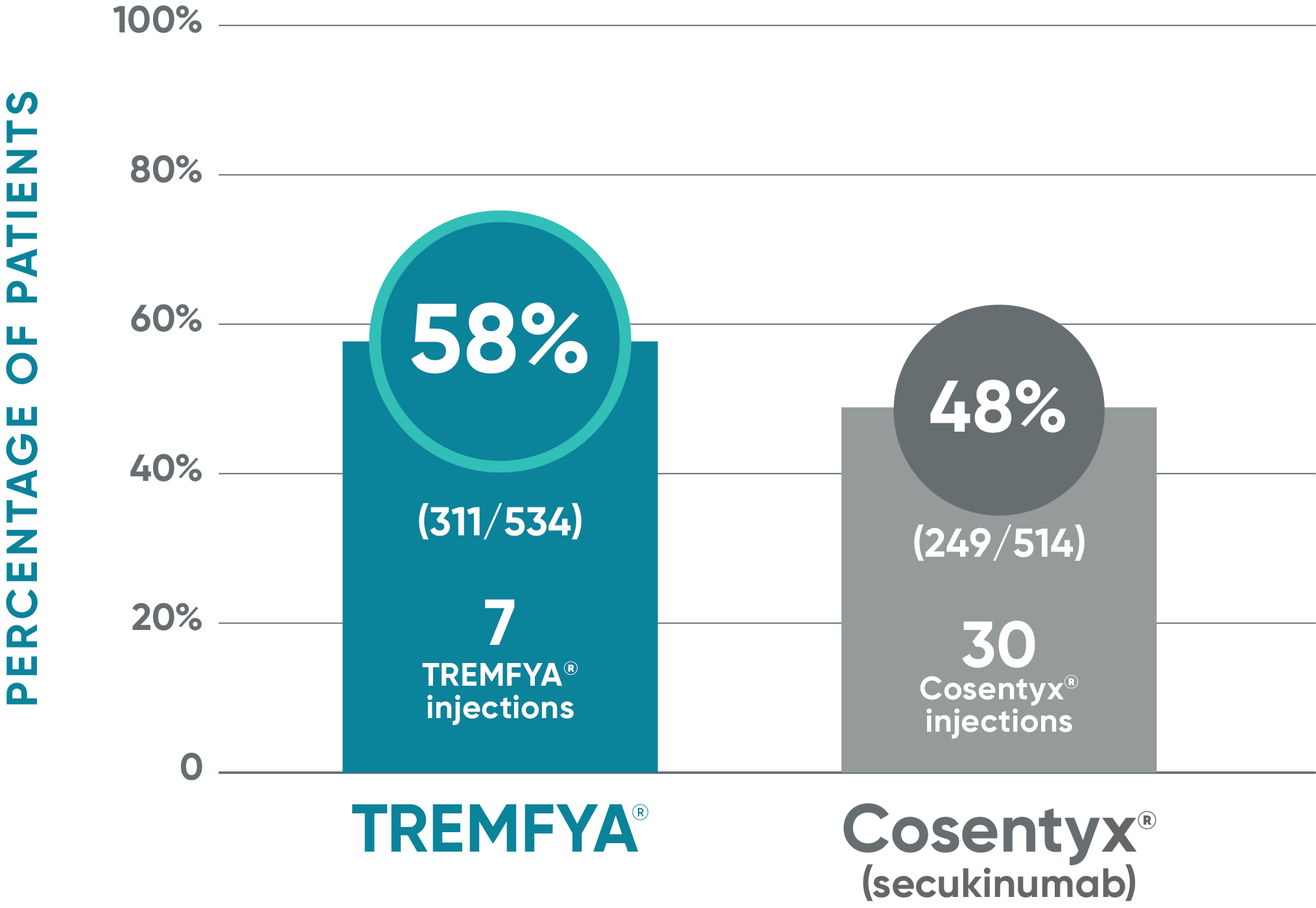
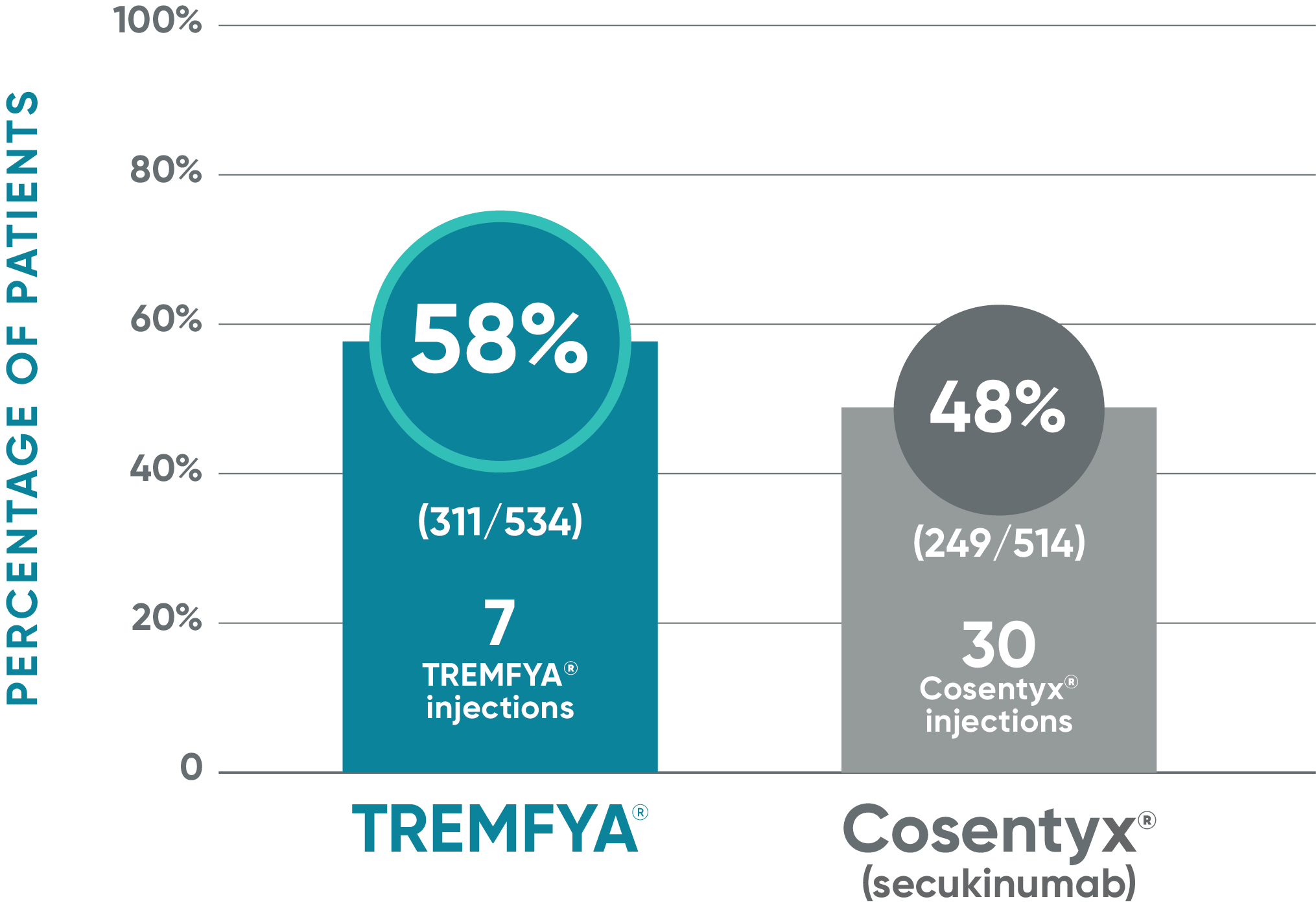
ECLIPSE: Major secondary endpoint at Week 48
VOYAGE pivotal trials' co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
Psoriasis Symptoms and Signs Diary (Week 16): Greater improvements in symptoms of psoriasis (itch, pain, stinging, burning, and skin tightness).3
Secondary endpoint at Week 48
- IGA 0/1: TREMFYA® 85% (454/534), Cosentyx® 75% (385/514)1,2
Nonresponder imputation (NRI) methods were used for analysis.
Results based on ECLIPSE: a single study of TREMFYA® vs Cosentyx®.
*PASI 100=proportion of patients who achieved 100% or more reduction (or improvement) in PASI score from baseline.
References: 1. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. 2. Data on file. Janssen Biotech, Inc. 3. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
PASI 90 response with TREMFYA® at Week
48 by baseline body weight quartile1
ECLIPSE: Post hoc analysis of body weight—PASI 90 at Week 48




VOYAGE pivotal trials' co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
Psoriasis Symptoms and Signs Diary (Week 16): Greater improvements in symptoms of psoriasis (itch, pain, stinging, burning, and skin tightness).2
Nonresponder imputation (NRI) methods were used.
This is a post hoc analysis; statistical significance has not been established.
The quartile cutoffs are based on the overall population (not by treatment group).
References: 1. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA:Janssen Biotech, Inc. 3. Data on file. Janssen Biotech, Inc.
PASI 90 response with TREMFYA® at
Week 48 by baseline BMI category1
ECLIPSE: Prespecified subgroup analysis of BMI category—PASI 90 at Week 48




VOYAGE pivotal trials' co-primary endpoints at Week 16 (NRI)2,3:
VOYAGE 1—PASI 90: TREMFYA® 73% (241/329), placebo 3% (5/174) (P<0.001). IGA 0/1: TREMFYA® 85% (280/329), placebo 7% (12/174) (P<0.001). VOYAGE 2—PASI 90: TREMFYA® 70% (347/496), placebo 2% (6/248) (P<0.001). IGA 0/1: TREMFYA® 84% (417/496), placebo 8% (21/248) (P<0.001).
Psoriasis Symptoms and Signs Diary (Week 16): Greater improvements in symptoms of psoriasis (itch, pain, stinging, burning, and skin tightness).2
Nonresponder imputation (NRI) methods were used.
This is a prespecified subgroup analysis; statistical significance has not been established.
References: 1. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. 2. TREMFYA® (guselkumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 3. Data on file. Janssen Biotech, Inc.




