Watch TREMFYA® mechanism of action
Plaque psoriasis was originally thought to be a disorder driven primarily by dysregulated skin cell proliferation. However, years of research have revealed that plaque psoriasis is actually a disorder of the immune system, driven primarily by dysregulated T cells.
Today, biologic agents are one of the treatments used to treat plaque psoriasis. The first biologic agents focused on controlling broad inflammatory cytokines like tumor necrosis factor (TNF).
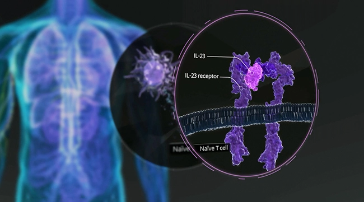
However, subsequent biologics began to target specific T cell pathways. These pathways include two main targets, regulatory cytokines, such as interleukin 23 and interleukin 12, and effector cytokines such as interleukin 17A and interleukin 17F. Recent findings suggest that the IL-23 Th17 axis is one of the key pathways involved in the pathogenesis of psoriasis.
Looking more closely at IL-23, interaction of this cytokine with its receptor triggers the differentiation, proliferation, and survival of these T cells. The cytokines produced by these T cells stimulate inflammation and keratinocyte proliferation. IL-23 has become an important target in developing biologic therapies for plaque psoriasis.
TREMFYA® (guselkumab) is an interleukin 23 blocker indicated for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, as well as the treatment of adult patients with active psoriatic arthritis.
TREMFYA® is a human monoclonal IgG1 lambda antibody that selectively binds to the p19 subunit of IL-23 and inhibits its interaction with the IL-23 receptor, thereby inhibiting the release of pro inflammatory cytokines and chemokines.
In evaluated subjects with plaque psoriasis, TREMFYA® reduced serum levels of IL-17A, IL-17F, and IL-22 relative to pre-treatment levels based on exploratory analysis of the pharmacodynamic markers. The relationship between these pharmacodynamic markers and the mechanisms by which TREMFYA® exerts its clinical effects is unknown. TREMFYA® was the first biologic to selectively target and bind with high specificity and affinity to IL-23.
INDICATIONS
TREMFYA® (guselkumab) is indicated for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
TREMFYA® is indicated for the treatment of adults with active psoriatic arthritis.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
TREMFYA® is contraindicated in patients with a history of serious hypersensitivity reaction to guselkumab or to any of the excipients.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Serious hypersensitivity reactions including anaphylaxis have been reported with post market use of TREMFYA®. Some cases required hospitalization. If a serious hypersensitivity reaction occurs, discontinue TREMFYA® and initiate appropriate therapy.
Infections
TREMFYA® may increase the risk of infection. Treatment with TREMFYA® should not be initiated in patients with a clinically important active infection until the infection resolves or is adequately treated.
Consider the risks and benefits of treatment prior to prescribing TREMFYA® in patients with a chronic infection or history of recurrent infection. Instruct patients receiving TREMFYA® to seek medical help if signs or symptoms of clinically important chronic of acute infection occur. If a patient develops a clinically important or serious infection or is not responding to standard therapy, closely monitor and discontinue TREMFYA® until the infection resolves.
Pre-Treatment Evaluation for Tuberculosis (TB)
Evaluate patients for TB infection prior to initiating treatment with TREMFYA®. Initiate treatment of latent TB prior to administering TREMFYA®. Monitor patients for signs and symptoms of active TB during and after TREMFYA® treatment. Do not administer TREMFYA® to patients with active TB infection.
Immunizations
Prior to initiating TREMFYA®, consider completion of all age appropriate immunizations according to current immunization guidelines. Avoid the use of live vaccines in patients treated with TREMFYA®.
ADVERSE REACTIONS
Most common (≥1%) adverse reactions associated with TREMFYA® include upper respiratory infections, headache, injection site reactions, arthralgia, bronchitis, diarrhea, gastroenteritis, tinea infections, and herpes simplex infections.
The overall safety profile observed in patients with psoriatic arthritis is generally consistent with the safety profile in patients with plaque psoriasis, with the addition of bronchitis and neutrophil count decreased.
Please read the full Prescribing Information and Medication Guide for TREMFYA®. Provide the Medication Guide to your patients and encourage discussion.
Demonstration video: how to inject TREMFYA® with One-Press injector
Are you ready to inject TREMFYA®, also known as guselkumab, using the One-Press patient-controlled injector? TREMFYA® is given as a 100-mg injection under your skin that you only need to take once every eight weeks after two starter doses at Weeks 0 and 4.
Your first self injection may be performed at your healthcare provider’s office so he or she can show you the right way to give yourself injections under the skin. After this training, and with your healthcare provider’s approval, you may be able to inject at home.
If you do inject at home, remember that TREMFYA® must be stored in the refrigerator at 36° to 46° Fahrenheit. Do not freeze your One-Press injector. Do not shake your One-Press injector. Keep your One-Press injector and all medicines out of reach of children and keep it in the original carton to protect it from light and physical damage.
What follows are instructions for how to inject TREMFYA® using the One-Press injector. But first, carefully read the Instructions for Use and Medication Guide enclosed in the carton before you or your caregiver injects TREMFYA® and each time you get a refill.
This video will help you get comfortable with using One-Press injector to self inject TREMFYA® as prescribed by your healthcare provider. It’s important that you don’t try to inject yourself until your healthcare provider has walked you through the whole process.
Let’s get started.
Let’s first familiarize ourselves with the One-Press injector itself. On the left side of the screen is the One-Press injector before use. You see the handle, the teal body of the device, the window where you can inspect the liquid, the needle guard, and the bottom cap over the needle guard. Do not remove the bottom cap until you are ready to inject. On the right side of the screen is the One-Press injector after use. The One-Press injector is a patient-controlled device, so the medication injects as you push the handle. You can do this at a speed that is comfortable for you.
Now you’ll need a few supplies to prepare for your injection: one alcohol swab, one cotton ball or gauze pad, one adhesive bandage, and one sharps container. These items are not included with the One-Press injector.
Before you inject, remove the carton with your One-Press injector from the fridge. Leave the One-Press injector in the carton and let it sit on a flat surface at room temperature for at least 30 minutes before you inject. Do not attempt to warm the medicine any other way. Make sure you check the expiration date on the carton and do not use the One-Press injector if the date has passed. Do not use the One-Press injector if the seal on the carton is broken. If you find this or any other damage to the carton, call your healthcare provider or pharmacist to get a new One-Press injector.
Next, choose one of these injection sites. We recommend the front of your thighs. But you can also choose an area on your lower stomach, but never within the two inches around your belly button. And if you have someone giving you the injection, the back of one of your upper arms is also an option. Remember, do not inject into skin that is tender, bruised, red, scaly, hard, thick, or is affected by psoriasis.
Wash your hands well with soap and warm water. Next, clean your chosen injection site by wiping with the alcohol swab, then allowing it to dry. Do not touch, fan, or blow on the injection site after you’ve cleaned it.
Take your One-Press injector out of the carton. Take a look at the liquid in the viewing window on the One-Press injector. It should be clear to slightly yellow and may contain tiny white or clear particles. You may also see one or more air bubbles. All of this is completely normal. Do not inject if the liquid is cloudy, discolored or has large particles. Call your healthcare provider or pharmacist and ask for a new One-Press injector.
So it’s been at least 30 minutes since you took the carton out of the fridge, right? Now, take the One-Press injector and pull off the bottom cap. Make sure you keep your hands away from the needle guard after the cap is removed. It is normal to see a few drops of liquid. Make sure you complete your injection within five minutes of removing the cap. Do not put the cap back on. This could damage the needle. Do not use the One-Press injector if it’s dropped after the cap has been removed. If this happens, call your healthcare provider or pharmacist to get a new prefilled One-Press injector.
Place the One-Press injector straight onto the skin at the injection site. Push the handle all the way down until the teal body is not visible. This is very important. Do not lift the One-Press injector during the injection. If you do, the needle guard will lock, showing a yellow band, and you will not get the full dose. You may hear a click when the injection begins. Keep pushing. If you feel resistance, keep pushing. This is normal. The medication injects as you push. Do this at a speed that is comfortable for you.
Confirm that your injection is complete. The injection is complete when the teal body is not visible and you cannot press the handle down anymore. You may hear a click. Then lift straight up from the injection site. The yellow band will tell you that the needle guard is locked.
Now, all you’ve got to do is a bit of cleanup. First, put your used One-Press injector in a sharps disposal container right away after use. Do not throw away or dispose of your One-Press injector in your household trash and do not recycle your used sharps disposal container. Make sure you dispose of the bin and its contents as instructed by your healthcare provider, nurse, or pharmacist. For more information, please read the section on disposing of TREMFYA® One-Press injector in the Instructions for Use.
There may be a small amount of blood or liquid at the injection site. Hold pressure over your skin using a cotton ball or gauze pad until any bleeding stops, but do not rub the injection site. If needed, cover the injection site with a bandage.
Congrats! Your self injection with TREMFYA® and One-Press is done. When it comes time for your next injection, this video can serve as a great refresher if you’re feeling a little rusty about the process. So definitely come back and watch it again. Remember to read the Instructions for Use before using your One-Press injector and each time you get a new one. There may be new information.
Please reach out to your healthcare provider for any questions you may have about your treatment. If you need additional injection support or would like information about a program to safely return your used devices at no cost to you, you can connect with a TREMFYA withMe Guide at 1-833-WITHME1. That’s 948-4631, Monday through Friday, from 8 AM to 11 PM Eastern time.
Thanks for clicking with TREMFYA® and One-Press.
TREMFYA® (guselkumab) is not for everyone; only your doctor can decide if it’s right for you. Do not use if you are allergic to TREMFYA®. TREMFYA® is a prescription medicine that may cause serious side effects, including serious allergic reactions and infections. TREMFYA® affects your immune system. It may increase your risk of infections and lower your ability to fight them. Please read the Important Safety Information and the Medication Guide for TREMFYA® to learn more about these and other risks for TREMFYA®. Discuss any questions you have with your doctor.
Demonstration video: how to inject TREMFYA® with prefilled syringe
Getting comfortable with taking TREMFYA® (guselkumab)
Carefully read the Instructions for Use and Medication Guide enclosed with your prefilled syringe before you or a caregiver administer TREMFYA® and each time you get a refill.
Hello there and welcome to your easy-to-follow overview of self injecting TREMFYA®. If you’re watching this video, you’ve already received training from your healthcare professional and he or she has decided you’re ready to begin self administering TREMFYA®. That’s great. This video can help you feel even more comfortable with self injection.
Remember, it’s important that you don’t try to inject yourself until your healthcare provider has shown you the correct way to give yourself an injection.
Before we do any actual injecting, let’s first familiarize ourselves with the syringe itself. When you first take it out of the package, it’ll look like this, complete with plunger, safety guard, finger flange, body, viewing window, and the needle cover. Do not hold or pull the plunger at any time and do not remove the needle cover until you are ready to inject TREMFYA®. After you inject TREMFYA®, it’ll look like this. The safety guard will cover the needle and lock into place.
Next, you’ll need to gather a few basic supplies: an alcohol swab, a cotton ball or gauze pad, an adhesive bandage, and a sharps container to put your syringe in when you’re done. Now, take your carton of TREMFYA® out of the fridge. Keep the prefilled syringe in the carton and let it sit on a flat surface at room temperature for at least 30 minutes before use. Do not warm the prefilled syringe any other way.
Next, check the expiration date located right here on the back of the carton. If it’s expired, don’t use it. While you’re at it, check the carton to make sure it hasn’t been opened or tampered with. If it has, do not inject and call your healthcare provider or pharmacist for a refill.
TREMFYA® prefilled syringe is given as an injection under the skin. As far as injection sites go, you can use areas of the body like the front of your thigh or your lower stomach area. Just remember the two inch radius around your belly button must be avoided. If you’ve got someone there to help you out, the back of your upper arm is also an option. Make sure you don’t inject into skin that is tender, bruised, red, hard, thick, scaly, or affected by psoriasis.
Take a moment to wash your hands well with soap and warm water. Wipe your chosen injection site with an alcohol swab and allow it to dry. Now that you’ve got it clean and ready to go, do not touch, fan, or blow on the injection site after cleaning it.
Now take your TREMFYA® prefilled syringe out of the carton. Inspect the TREMFYA® prefilled syringe liquid in the viewing window. It should be clear to slightly yellow and may contain tiny white or clear particles. You may also see one or more air bubbles. This is normal. Do not inject if the liquid is cloudy or discolored or has large particles. Call your healthcare provider or pharmacist for a refill.
Now, let’s get started. Hold your prefilled syringe by the body and pull the needle cover straight off. It is normal to see a drop of liquid. Inject TREMFYA® within five minutes of removing the needle cover. Do not put the needle cover back on, as this may damage the needle or cause a needle stick injury. Do not touch the needle or let it touch any surface. And do not use a TREMFYA® prefilled syringe if it is dropped. Call your healthcare provider or pharmacist for a refill.
Place your thumb, index, and middle fingers directly under the finger flange as shown here. Do not touch the plunger or the area above the finger flange, as this may cause the needle safety device to activate. Use your other hand to pinch skin at the injection site. Position the syringe at about a 45 degree angle to the skin. It’s important to pinch enough skin to inject under the skin and not into the muscle.
Insert needle with a quick dart-like motion. See? Like this. Release the pinched skin and now use that hand to grasp the body of the prefilled syringe. Place your thumb from the opposite hand on the plunger and press the plunger all the way down until it stops. Release pressure from the plunger. The safety guard will cover the needle and lock into place, removing the needle from your skin.
Put your used TREMFYA® prefilled syringe in your FDA-cleared sharps disposal container right away after use. Do not throw away your TREMFYA® prefilled syringe in your household trash. And do not recycle your used sharps disposal container. For more information, see “How Should I Dispose of the Used Prefilled Syringe?” in the Instructions for Use for TREMFYA®.
Check the injection site. There may be a little blood or liquid where you gave yourself the injection. Just use the cotton ball or gauze pad to apply light pressure until bleeding stops. Do not rub the injection site. If needed, cover the injection site with a bandage.
Your injection is now complete.
Set a reminder in your calendar for your next injection. Call your healthcare provider to talk about any questions you may have. For additional assistance or to share your feedback, call 800-Janssen (800-526-7736).
Now that we’ve taken care of your injection, it’s time to hear some selected important safety information.
SELECTED IMPORTANT SAFETY INFORMATION
TREMFYA® is not for everyone; only your doctor can decide if it’s right for you. Do not use if you are allergic to TREMFYA®. TREMFYA® is a prescription medicine that may cause serious side effects, including serious allergic reactions and infections. TREMFYA® affects your immune system. It may increase your risk of infections and lower your ability to fight them. Please read the Important Safety Information located on this website and the Medication Guide for TREMFYA® to learn more about these and other risks for TREMFYA®. Discuss any questions you have with your doctor.
Learn about the TREMFYA®
molecular structure with
Dr. Gordon Lam
Hello, I’m Doctor Gordon Lam. I’m the Medical Director of Clinical Research for the Arthritis & Osteoporosis Consultants of the Carolinas and an affiliate of Atrium Health and the Wake Forest School of Medicine in Charlotte, North Carolina. I’ll be narrating this video and explaining the mechanism of action of guselkumab, including its binding to IL-23 and CD64.
Guselkumab is indicated for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Guselkumab is indicated for the treatment of adults with active psoriatic arthritis.
Guselkumab is the first fully human monoclonal antibody that specifically targets and inhibits interleukin 23 (IL-23) by binding to its p19 subunit.
IL-23 is known to be a key driver of several inflammatory diseases including plaque psoriasis and psoriatic arthritis. IL-23 is secreted by activated cells such as monocytes and dendritic cells and binds to the IL-23 receptor complex on target immune cells. This induces the production of other cytokines in several inflammatory disorders.
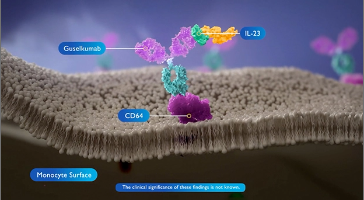
Guselkumab selectively binds to the p19 subunit of IL-23 and inhibits its interaction with the IL-23 receptor. This blocks the downstream signaling and release the pro-inflammatory cytokines, thereby reducing inflammation. Guselkumab shares similar in vitro pharmacologic profiles with other anti IL-23 antibody therapies. Additionally, guselkumab has been shown to bind to FC gamma receptor 1, also known as CD64. The clinical significance of these findings is not known.
CD64 is present on IL-23 secreting cells such as monocytes and macrophages. The majority of IL-23 is secreted by CD64 positive monocytes. The monoclonal antibody guselkumab has a wild type FC region that enables it to bind to CD64 on mononuclear phagocytes. Guselkumab is the only fully human, high affinity IL-23 inhibitor that simultaneously bind to CD64 at its FC region and binds IL-23 at its antigen binding domain. The clinical significance of these findings is not known.
Taken together, guselkumab’s presence may be enriched within the inflamed tissue microenvironment by binding to the CD64 and neutralizing IL-23. Further studies are warranted to generate additional evidence to support this hypothesis.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Guselkumab is contraindicated in patients with a history of serious hypersensitivity reaction to guselkumab or to any of the excipients.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, have been reported with postmarket use of guselkumab. Some cases required hospitalization. If a serious hypersensitivity reaction occurs, discontinue guselkumab and initiate appropriate therapy.
Infections
Guselkumab may increase the risk of infection. Treatment with guselkumab should not be initiated in patients with a clinically important active infection until the infection resolves or is adequately treated.
Consider the risks and benefits of treatment prior to prescribing guselkumab in patients with a chronic infection or history of recurrent infection. Instruct patients receiving guselkumab to seek medical help if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops a clinically important or serious infection, or is not responding to standard therapy, closely monitor and discontinue guselkumab until the infection resolves.
Pre-Treatment Evaluation for Tuberculosis
Evaluate patients for tuberculosis infection prior to initiating treatment with guselkumab. Initiate treatment of latent tuberculosis prior to administering guselkumab. Monitor patients for signs and symptoms of active tuberculosis during and after guselkumab treatment. Do not administer guselkumab to patients with active tuberculosis infection.
Immunizations
Prior to initiating guselkumab, consider completion of all age-appropriate immunizations according to current immunization guidelines. Avoid use of live vaccines in patients treated with guselkumab.
ADVERSE REACTIONS
Most common (≥1%) adverse reactions associated with guselkumab include upper respiratory infections, headache, injection site reactions, arthralgia, bronchitis, diarrhea, gastroenteritis, tinea infections, and herpes simplex infections.
The overall safety profile observed in patients with psoriatic arthritis is generally consistent with the safety profile in patients with plaque psoriasis, with the addition of bronchitis and neutrophil count decreased.
Please read the full Prescribing Information and Medication Guide for guselkumab. Provide the Medication Guide to your patients and encourage discussion.
TREMFYA® Patient Stories: Suzana—Impact of PsA Symptoms
Hi, my name is Suzana and I live in McHenry, Illinois, close to the border of Wisconsin.
How do I spend more time with my children? They were little. One was in fourth grade, one was in seventh grade, and we had just moved and they were struggling and they needed me more than anything, probably more than any time in their lives.
I was having swelling and joint pain. And I was just struggling to like make dinner every day because of my joint symptoms. And that was when I really took action. I’m not going to take this sitting down. I actually found another rheumatologist who was familiar with TREMFYA®. He mentioned about it and we decided to try it and see what it could do for my condition of active psoriatic arthritis.
INDICATION
What is TREMFYA® (guselkumab)?
TREMFYA® is a prescription medicine used to treat adults with active psoriatic arthritis.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about TREMFYA®? TREMFYA® is a prescription medicine that may cause serious side effects, including:
- Serious Allergic Reactions. Stop using TREMFYA® and get emergency medical help right away if you develop any of the following symptoms of a serious allergic reaction:
- fainting, dizziness, feeling lightheaded (low blood pressure)
- swelling of your face, eyelids, lips, mouth, tongue, or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
- itching
- Infections. TREMFYA® may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with TREMFYA® and may treat you for TB before you begin treatment with TREMFYA® if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with TREMFYA®.
Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- muscle aches
- weight loss
- cough
- warm, red, or painful skin or sores on your body different from your psoriasis
- diarrhea or stomach pain
- shortness of breath
- blood in your phlegm (mucus)
- burning when you urinate or urinating more often than normal
Do not take TREMFYA® if you have had a serious allergic reaction to guselkumab or any of the ingredients in TREMFYA®.
Before using TREMFYA®, tell your healthcare provider about all of your medical conditions, including if you:
- have any of the conditions or symptoms listed in the section “What is the most important information I should know about TREMFYA®?”
- have an infection that does not go away or that keeps coming back.
- have TB or have been in close contact with someone with TB.
- have recently received or are scheduled to receive an immunization (vaccine). You should avoid receiving live vaccines during treatment with TREMFYA®.
- are pregnant or plan to become pregnant. It is not known if TREMFYA® can harm your unborn baby.
Pregnancy Registry: If you become pregnant during treatment with TREMFYA®, talk to your healthcare provider about registering in the pregnancy exposure registry for TREMFYA®. You can enroll by visiting www.mothertobaby.org/
ongoing-study/tremfya-guselkumab, by calling 1-877-311-8972, or emailing [email protected]. The purpose of this registry is to collect information about the safety of TREMFYA® during pregnancy.
- are breastfeeding or plan to breastfeed. It is not known if TREMFYA® passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of TREMFYA®?
TREMFYA® may cause serious side effects. See “What is the most important information I should know about TREMFYA®?”
The most common side effects of TREMFYA® include: respiratory tract infections, headache, injection site reactions, joint pain (arthralgia), diarrhea, stomach flu (gastroenteritis), fungal skin infections, herpes simplex infections, and bronchitis.
These are not all the possible side effects of TREMFYA®. Call your doctor for medical advice about side effects.
Use TREMFYA® exactly as your healthcare provider tells you to use it.
Please read the full Prescribing Information, including Medication Guide, for TREMFYA® and discuss any questions that you have with your doctor.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Dosage Forms and Strengths: TREMFYA® is available in a 100 mg/mL prefilled syringe and One-Press patient-controlled injector for subcutaneous injection, a 200 mg/2 mL prefilled syringe and prefilled pen (TREMFYA® PEN) for subcutaneous injection, and a 200 mg/20 mL (10 mg/mL) single dose vial for intravenous infusion.
cp-82626v7
TREMFYA® Patient Stories: Humberto—Impact of PsA Symptoms
My name is Humberto Macias. I live in Orange County, and I work every day. Some days long hours, some days not much. But that’s who I am.
I have plaque psoriasis in my skin. My knees were painful, stiff, and swollen. I couldn’t do certain exercises. Bending my knees was painful. Going upstairs or downstairs, it was painful on my knees.
I started taking TREMFYA® for my active PsA and my joint pain, stiffness, and swelling were significantly reduced at 24 weeks. The use of my knees has been improved and I can go upstairs, downstairs, walk with less joint symptoms.
If anyone who might be suffering from active psoriatic arthritis asks you for advice, tell them to be their own cheerleader and make the best decisions for them. Patients should ask doctors if TREMFYA® can be an appropriate treatment option for them.
INDICATION
What is TREMFYA® (guselkumab)?
TREMFYA® is a prescription medicine used to treat adults with active psoriatic arthritis.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about TREMFYA®? TREMFYA® is a prescription medicine that may cause serious side effects, including:
- Serious Allergic Reactions. Stop using TREMFYA® and get emergency medical help right away if you develop any of the following symptoms of a serious allergic reaction:
- fainting, dizziness, feeling lightheaded (low blood pressure)
- swelling of your face, eyelids, lips, mouth, tongue, or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
- itching
- Infections. TREMFYA® may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with TREMFYA® and may treat you for TB before you begin treatment with TREMFYA® if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with TREMFYA®.
Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- muscle aches
- weight loss
- cough
- warm, red, or painful skin or sores on your body different from your psoriasis
- diarrhea or stomach pain
- shortness of breath
- blood in your phlegm (mucus)
- burning when you urinate or urinating more often than normal
Do not take TREMFYA® if you have had a serious allergic reaction to guselkumab or any of the ingredients in TREMFYA®.
Before using TREMFYA®, tell your healthcare provider about all of your medical conditions, including if you:
- have any of the conditions or symptoms listed in the section “What is the most important information I should know about TREMFYA®?”
- have an infection that does not go away or that keeps coming back.
- have TB or have been in close contact with someone with TB.
- have recently received or are scheduled to receive an immunization (vaccine). You should avoid receiving live vaccines during treatment with TREMFYA®.
- are pregnant or plan to become pregnant. It is not known if TREMFYA® can harm your unborn baby.
Pregnancy Registry: If you become pregnant during treatment with TREMFYA®, talk to your healthcare provider about registering in the pregnancy exposure registry for TREMFYA®. You can enroll by visiting www.mothertobaby.org/
ongoing-study/tremfya-guselkumab, by calling 1-877-311-8972, or emailing [email protected]. The purpose of this registry is to collect information about the safety of TREMFYA® during pregnancy.
- are breastfeeding or plan to breastfeed. It is not known if TREMFYA® passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of TREMFYA®?
TREMFYA® may cause serious side effects. See “What is the most important information I should know about TREMFYA®?”
The most common side effects of TREMFYA® include: respiratory tract infections, headache, injection site reactions, joint pain (arthralgia), diarrhea, stomach flu (gastroenteritis), fungal skin infections, herpes simplex infections, and bronchitis.
These are not all the possible side effects of TREMFYA®. Call your doctor for medical advice about side effects.
Use TREMFYA® exactly as your healthcare provider tells you to use it.
Please read the full Prescribing Information, including Medication Guide, for TREMFYA® and discuss any questions that you have with your doctor.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Dosage Forms and Strengths: TREMFYA® is available in a 100 mg/mL prefilled syringe and One-Press patient-controlled injector for subcutaneous injection, a 200 mg/2 mL prefilled syringe and prefilled pen (TREMFYA® PEN) for subcutaneous injection, and a 200 mg/20 mL (10 mg/mL) single dose vial for intravenous infusion.
cp-82626v7
TREMFYA withMe Dedicated Nurse Guide*
So if you’ve never had a biologic and you’re trying TREMFYA® for the first time, I highly recommend calling TREMFYA withMe. They guide you through everything and they make it so less scary.
Direct communication to somebody who’s going to assist the patient with the medication. I’ve been with TREMFYA@ for a year and a half and I receive a phone call from a member of the team maybe every three months because they want to make sure I’m okay.
So you dictate like the…frequency of meeting with them. And so if you hang up with them and you’re like, I don’t really understand this, you can message them and get back on with them again or email them.
And I have a person by the name of Susan, and she follows up with me. How is my treatment? How’s TREMFYA®? How’s everything with the program? And this is TREMFYA withMe and I have it right here on my phone. TREMFYA withMe, Susan.
What is a specialty pharmacy?
So a specialty pharmacy is a pharmacy [that] handles, just like what it sounds like, special drugs. So special medications. So we’re talking costly medications or this is not like your normal everyday prescription.
They deal with drugs that aren’t readily available in a regular pharmacy, like for blood pressure or cholesterol. Um, because they need special handling.
Specialty pharmacies [are] very easy to deal with [in] a phone call. Then they make arrangements for me to receive the medication at any address, any place I want to. In my particular case, I received the medications at work because I’m there at normal, regular hours.
TREMFYA withMe Cost Support
If cost is a big factor for your, make sure you look into the TREMFYA withMe Savings Program.
On TREMFYA@ they offer a patient savings card if you have commercial insurance.
Very reasonable.
I was actually approached with a number that was kind of out of reach for me. It didn’t fit my family budget. Um, but I received information about the TREMFYA withMe Savings Program, which helped a lot.
It’s very simple, very easy, very easy to apply. TREMFYA@ has a very good support system. I was able to qualify easily just by filling out a form online. Almost like ordering pizza. Pizza can be expensive.
If you’re worried about cost, contact TREMFYA withMe and find out what options you have.