IN ADULT PATIENTS WITH ACTIVE PSORIATIC ARTHRITIS (PsA)
IN ADULT PATIENTS WITH ACTIVE PSORIATIC ARTHRITIS (PsA) OR MODERATE TO SEVERE PLAQUE PSORIASIS (PsO)
Consistent response rates from dose to dose1

of patients maintained clinical improvement at each 8-week dosing interval, assessed through Week 52
DISCOVER 2: Maintenance of cDAPSA MCII* at each dosing interval†
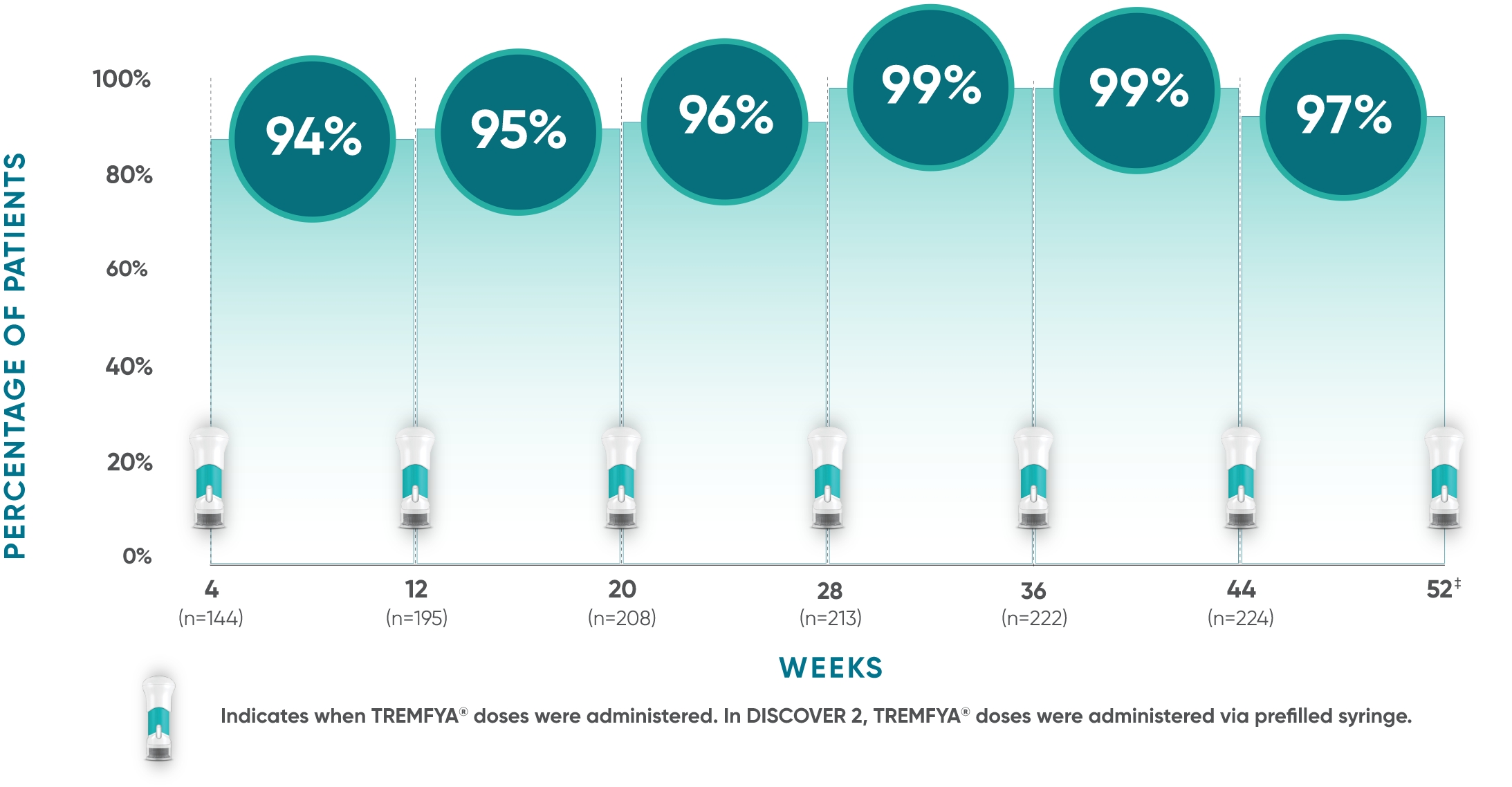

Indicates when TREMFYA® doses were administered. In DISCOVER 2, TREMFYA® doses were administered via prefilled syringe.
These endpoints were not adjusted for multiplicity. Therefore, statistical significance has not been established.
This post hoc analysis evaluated consistency of improvement at start and end of each 8-week dosing interval in bio-naïve patients (n=248).§
cDAPSA is the sum of 4 domain scores that reflect PsA disease activity: swollen joint count, tender joint count, patient assessment of pain severity, and patient global assessment of arthritis.
cDAPSA=clinical Disease Activity Index for PsA; MCII=minimal clinically important improvements; q8w=every 8 weeks.
*MCII is defined as the smallest change in measurement that signifies an important improvement at the individual patient level.
†The same patients may not have responded at each time interval.
‡After Week 52, efficacy assessments of DISCOVER 2 were scheduled for visits >8 weeks apart.
§Proportion of patients who achieved MCII at previous visit and maintained MCII at the subsequent TREMFYA® q8w dosing visit (eg, achieved at Week 4 and maintained at Week 12). Only patients with available data in the outcome of interest for each pair of visits were included in the analysis of that outcome.






