Induction Results
Both TREMFYA® SC and IV induction demonstrated clinical remission and endoscopic improvement at Week 121,2
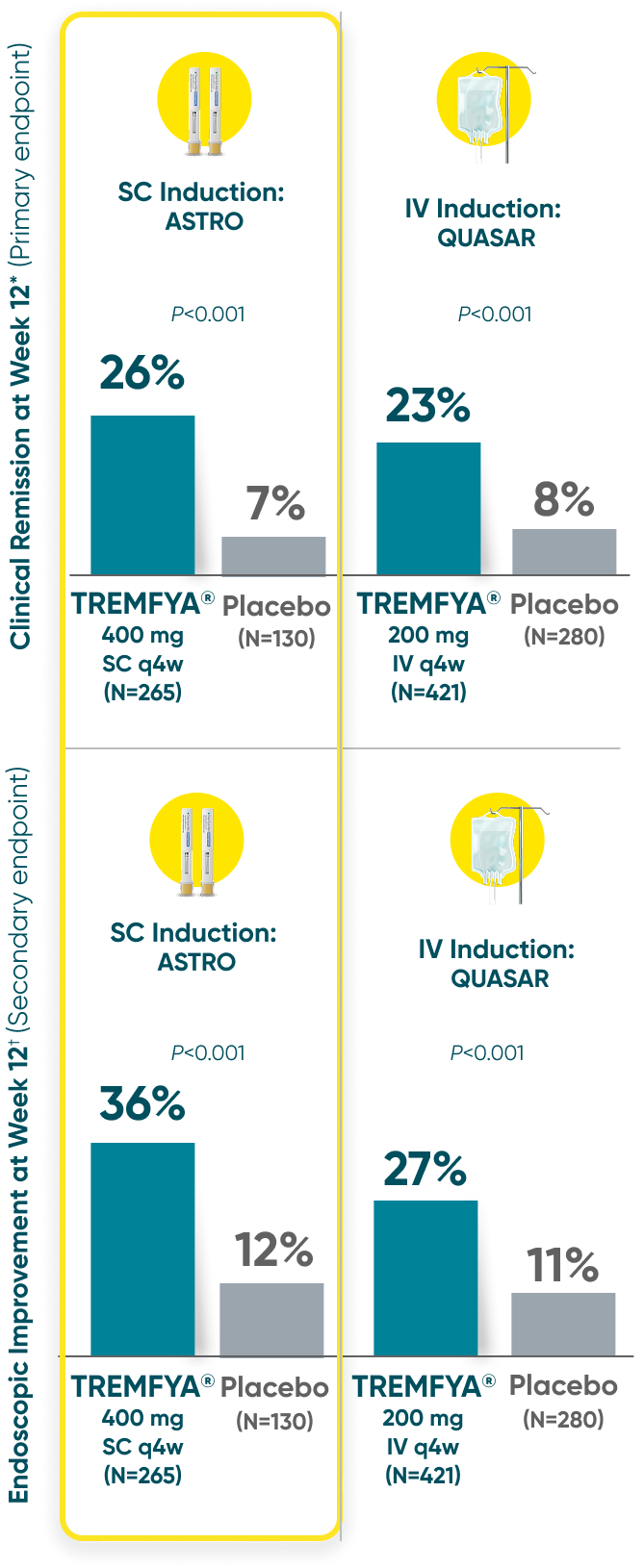
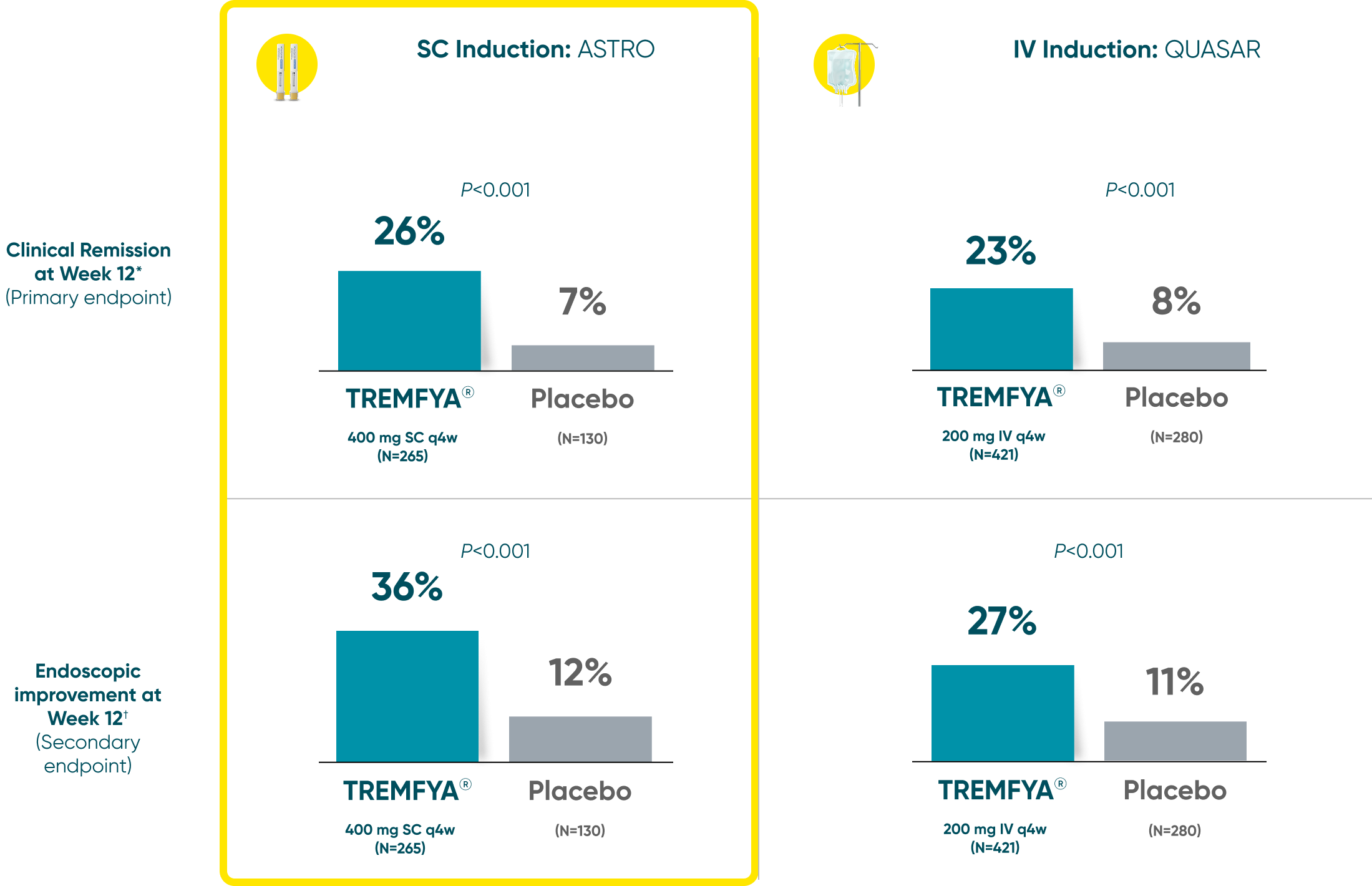
QUASAR and ASTRO were separate studies with separate endpoint assessments. Cross-trial comparisons are not meant to be made.
*Clinical remission: A stool frequency subscore of 0 or 1 and not increased from induction baseline, a rectal bleeding subscore of 0, and an endoscopy subscore of 0 or 1 with no friability.
†Endoscopic improvement: An endoscopy subscore of 0 or 1 with no friability.
IV=intravenous; MES=Mayo endoscopic subscore; q4w=every
4 weeks; q8w=every 8 weeks; SC=subcutaneous; UC=ulcerative colitis.
Symptomatic Response
TREMFYA® showed early separation from placebo in symptomatic response for both SC and IV induction1,2
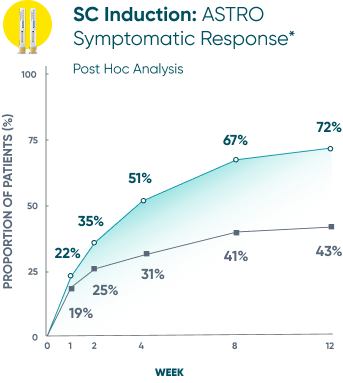
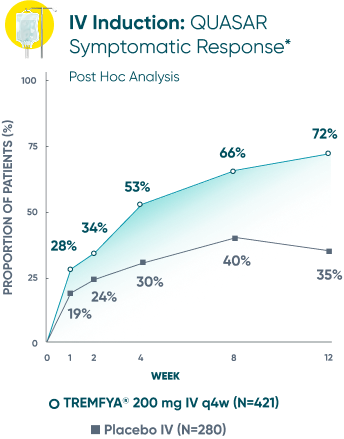
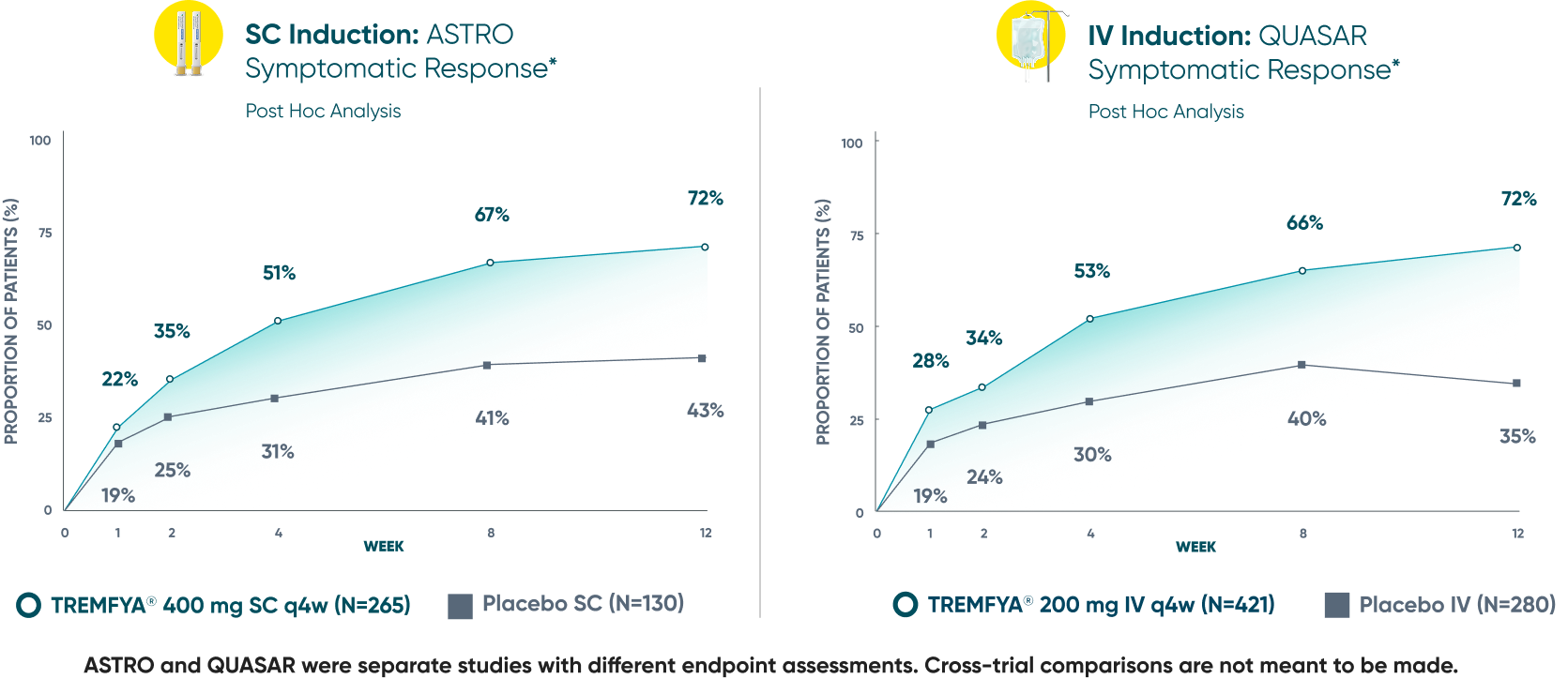
These are separate studies with different endpoint assessments. Cross-trial comparisons are not meant to be made.
DATA LIMITATION: Symptomatic response data through Week 12 was a post hoc analysis and not controlled for multiplicity in either study. No statistical significance can be made.
*Symptomatic response: A decrease from induction baseline in the symptomatic Mayo score by ≥30% and ≥1 point, with either a ≥1-point decrease from baseline in the rectal bleeding subscore or a rectal bleeding subscore of 0 or 1. The symptomatic Mayo score was defined as the sum of the stool frequency and the rectal bleeding subscores.2
IV=intravenous; q4w=every 4 weeks; SC=subcutaneous; UC=ulcerative colitis.
Endoscopic remission
(QUASAR STUDY)
Over 1/3 of all patients on TREMFYA® achieved complete endoscopic remission (MES=0) at
1 year1,2*†‡
QUASAR was a multicenter, randomized, double-blind, placebo-controlled induction and maintenance study
Endoscopic Remission at Week 442*†
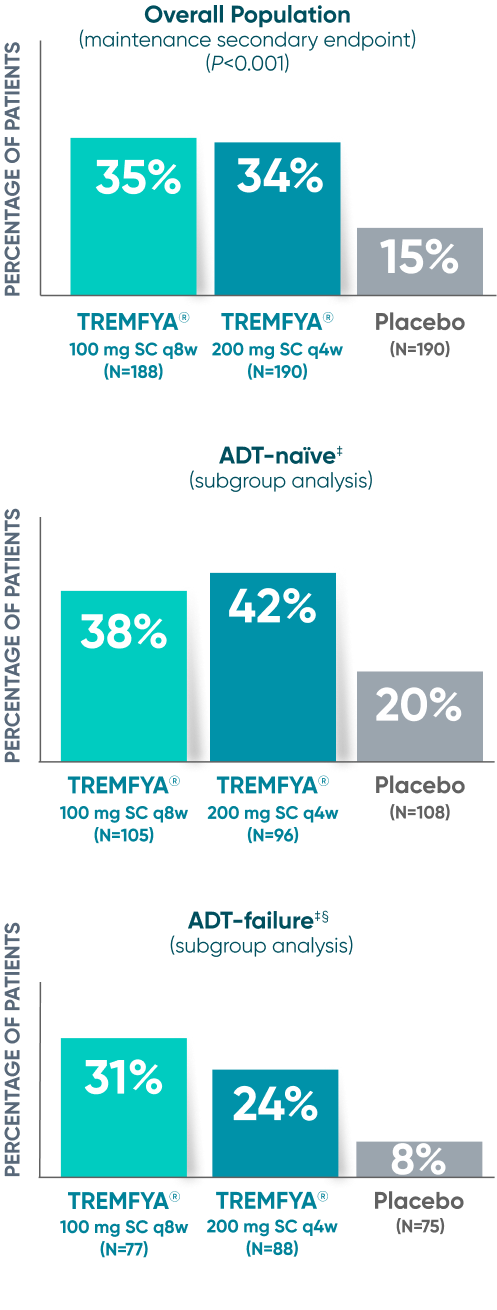
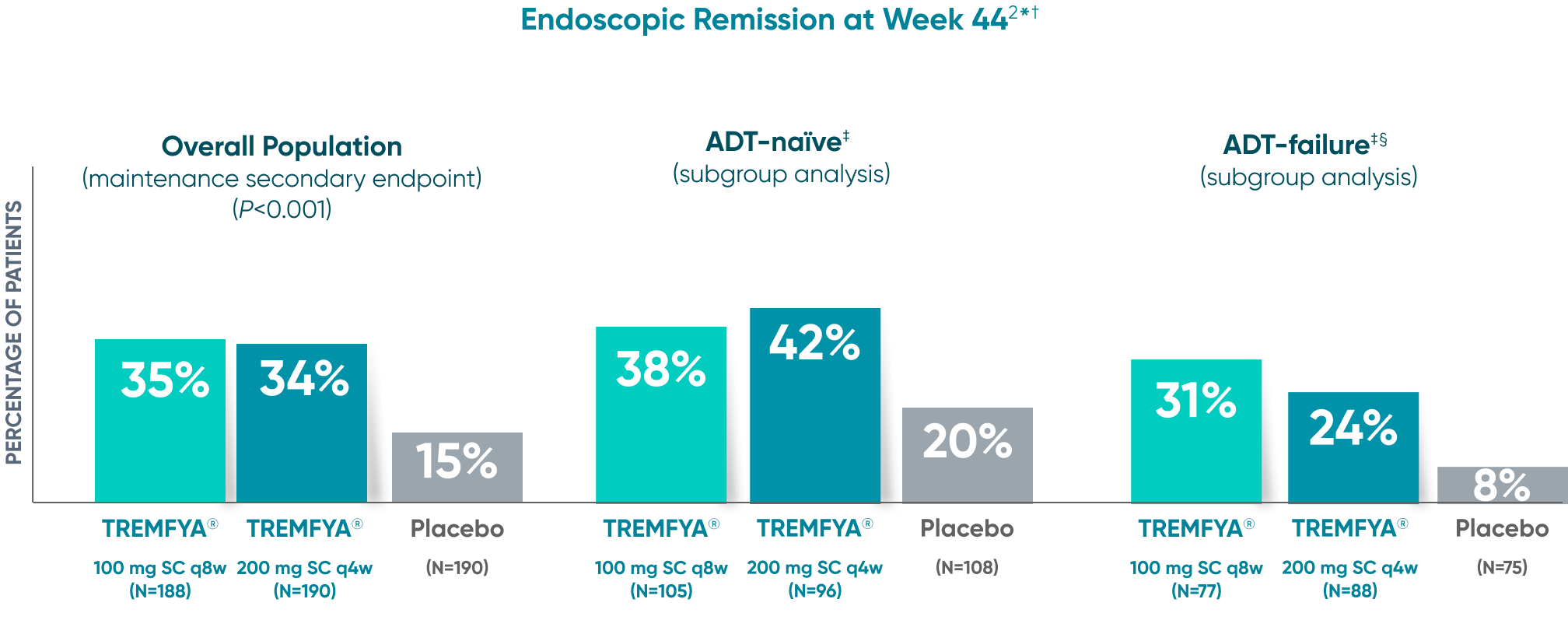
DATA LIMITATION SUBGROUP ANALYSIS: Endoscopic remission of the ADT-naïve and ADT-failure populations at 1 year were prespecified endpoints but not adjusted for multiplicity. No statistical significance can be made.
See the difference with endoscopic imagery in QUASAR2
From a real patient on TREMFYA® who was in endoscopic remission at Weeks 12 and 44
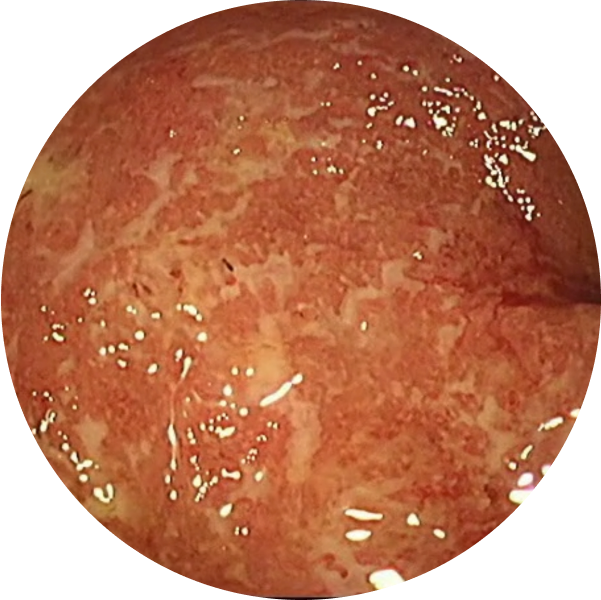
Before TREMFYA®
Week 0, MES=3
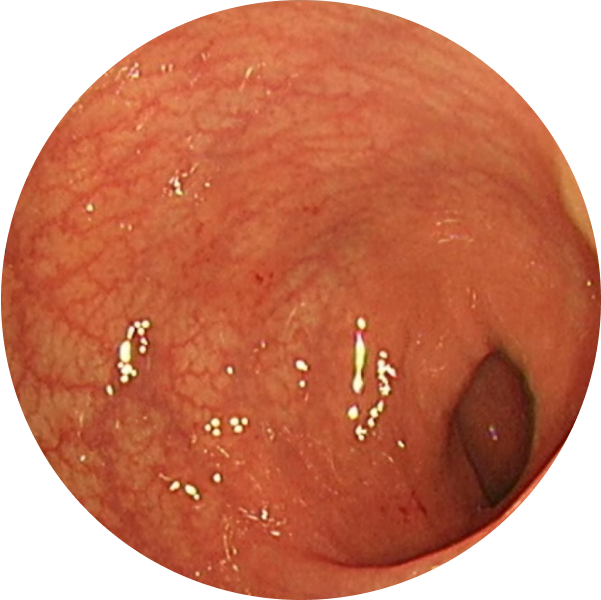
After TREMFYA®
Week 12, MES=0
Endoscopic Remission (MES=0) at Week 12 for TREMFYA® 200 mg IV q4w 15%
(63/421) and patients who received
placebo 5% (14/280)2*
DATA LIMITATION: Endoscopic remission at Week 12 was a prespecified endpoint but not adjusted for multiplicity. No statistical significance can be made.
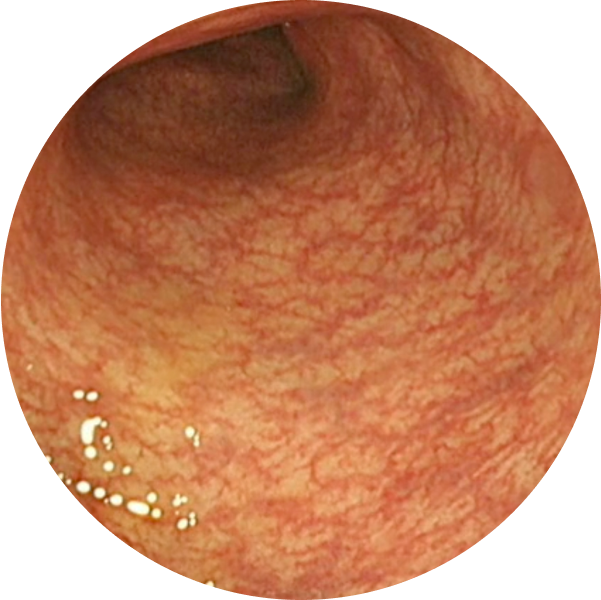
After TREMFYA®
Week 44, MES=0
Endoscopic Remission (MES=0) at Week 44 for TREMFYA® 200 mg SC q4w 34% (64/190), TREMFYA® 100 mg SC q8w 35% (65/188), and patients who received placebo 15% (29/190)1,2*
These images are of sections of an actual patient’s colon from the QUASAR study. Individual results may vary.
*Endoscopic remission (normalization) was defined as an endoscopy subscore of 0.1,
IV=intravenous; MES=Mayo endoscopic subscore; q4w=every 4 weeks; q8w=every 8 weeks; SC=subcutaneous.
*Week 44 of the maintenance study was defined as 1 year.
†Endoscopic remission (normalization) was defined as an endoscopy subscore of 0.2
‡ADTs in QUASAR: biologics (ie, TNF inhibitor, vedolizumab) or JAKi.
§Includes inadequate response, loss of response, or intolerance to biologic therapy (TNF blockers or vedolizumab) and/or a JAKi for UC.2
ADT=advanced therapy; JAKi=Janus kinase inhibitor; MES=Mayo Endoscopic Subscore; q4w=every 4 weeks; q8w=every 8 weeks; SC=subcutaneous; TNF=tumor necrosis factor;
OPEN-LABEL Long-Term Extension (LTE)
QUASAR LTE assessed efficacy and safety through 2 years2
All patients completing the maintenance study were eligible to enter the LTE2
Of the 188 patients randomized to TREMFYA® 100 mg SC q8w and 190 patients randomized to TREMFYA® 200 mg SC q4w in the maintenance study, 82.4% (155/188) and 77.9% (148/190), respectively, continued into the LTE as part of the LTE randomized analysis set. The LTE randomized full analysis set included all patients with an induction baseline modified Mayo score of 5 to 9 who were randomized in the maintenance study and did not experience a dose adjustment (or sham dose adjustment) and received at least 1 (partial or complete) dose of study intervention in the LTE2
Study was unblinded after final Week 44 maintenance analysis was completed. Patients randomized to placebo were discontinued after study unblinding and were not included in the open-label LTE efficacy analysis2
q4w=every 4 weeks; q8w=every 8 weeks.
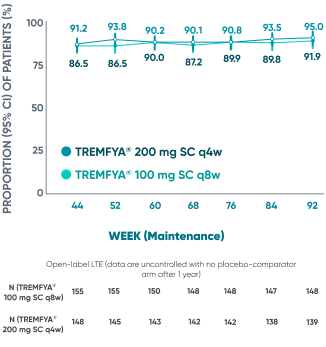
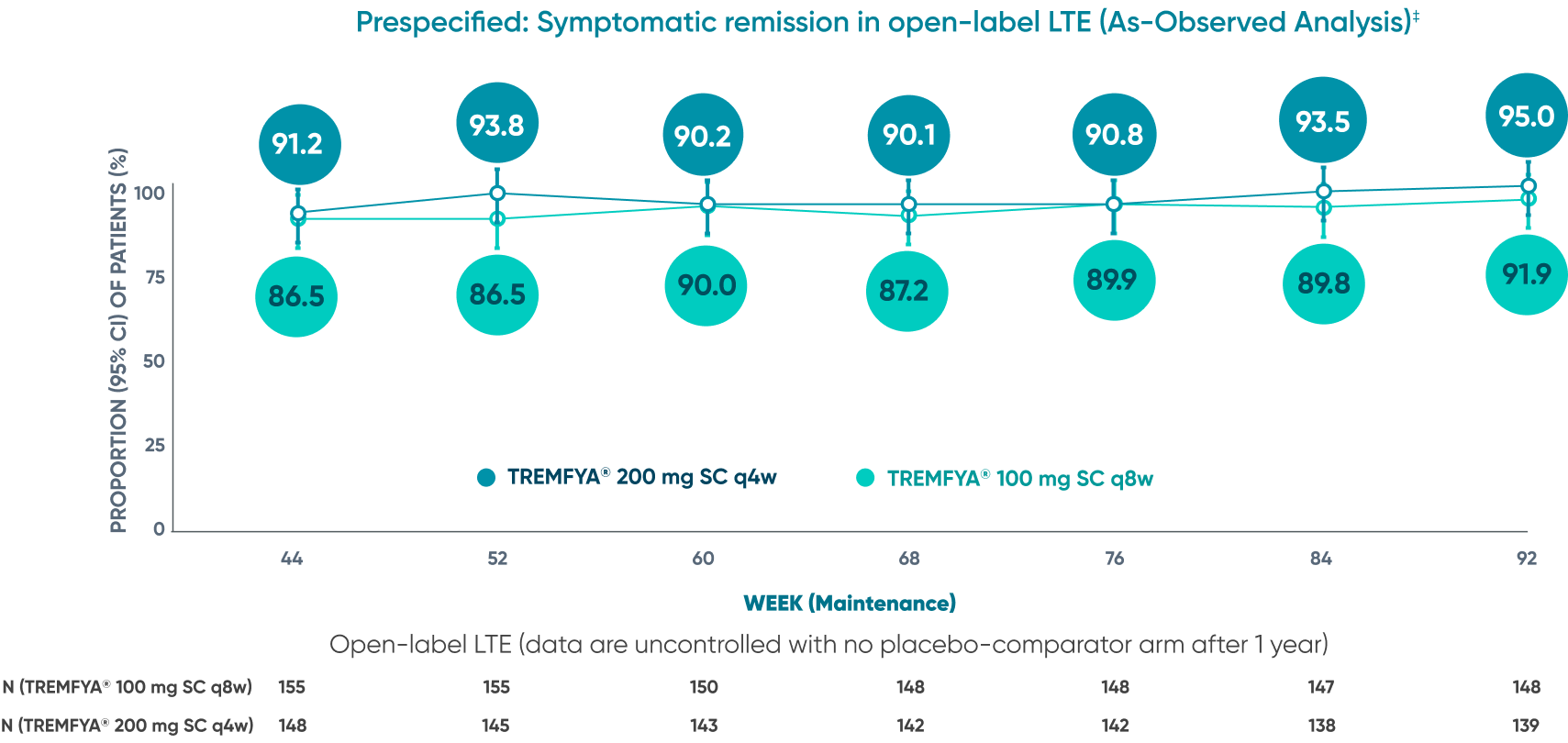
Majority of patients were in symptomatic remission at
1 year and through 2 years2*†‡
Of the patients who entered the LTE and remained in the study at Week 92
Symptomatic remission through 2 years*‡
Symptomatic remission at 1 year (QUASAR major secondary endpoint): TREMFYA® 200 mg SC q4w 69% (N=190, P<0.001), TREMFYA® 100 mg SC q8w 70% (N=188, P<0.001), and placebo SC 37% (N=190)
Prespecified: Symptomatic remission in open-label LTE (As-Observed Analysis)‡
Use the lowest effective recommended dosage to maintain therapeutic response.
DATA LIMITATION: Symptomatic remission through Week 92 during the LTE was prespecified but not controlled. Patients who dropped out of the LTE or who had missing data were not included in this as-observed analysis, which may increase the percentages of responders. No statistical significance can be made.
As-observed (AO) analysis: All observed data were analyzed exactly as collected, with missing data excluded from calculations for that visit, and regardless of whether patients experienced events during the study that could have affected the outcome measurement (such as discontinuing study intervention or having an ostomy or colectomy).
*Week 44 of the maintenance study was defined as 1 year. Week 92 of the LTE was defined as 2 years.
†LTE randomized full analysis set. Data are shown for participants who were in clinical response to TREMFYA® IV induction dosing and were randomized on entry into the maintenance study and did not experience a dose adjustment (including a sham dose adjustment) from Week 8 through Week 32.
‡Symptomatic remission was defined as a stool frequency subscore of 0 or 1 and not increased from baseline, and a rectal bleeding subscore of 0.
CI=confidence interval; IV=intravenous; LTE=long-term extension; q4w=every 4 weeks; q8w=every 8 weeks; SC=subcutaneous; UC=ulcerative colitis.
90% of patients who were in endoscopic improvement at
1 year were in endoscopic improvement at 2 years2*†‡
QUASAR endoscopic improvement in the open-label LTE (As-Observed Analysis)
Of the patients who entered the LTE and remained in the study at Week 92

Endoscopic improvement‡ at Week 44* of maintenance (Major secondary endpoint):
- TREMFYA® 200 mg SC q4w: 52% (98/190; P<0.001)
- TREMFYA® 100 mg SC q8w: 49.5% (93/188; P<0.001)
- Placebo SC: 19% (36/190)
QUASAR endoscopic improvement in the open-label LTE
(as-observed analysis)
Of the patients who entered the LTE and remained in the study at Week 92
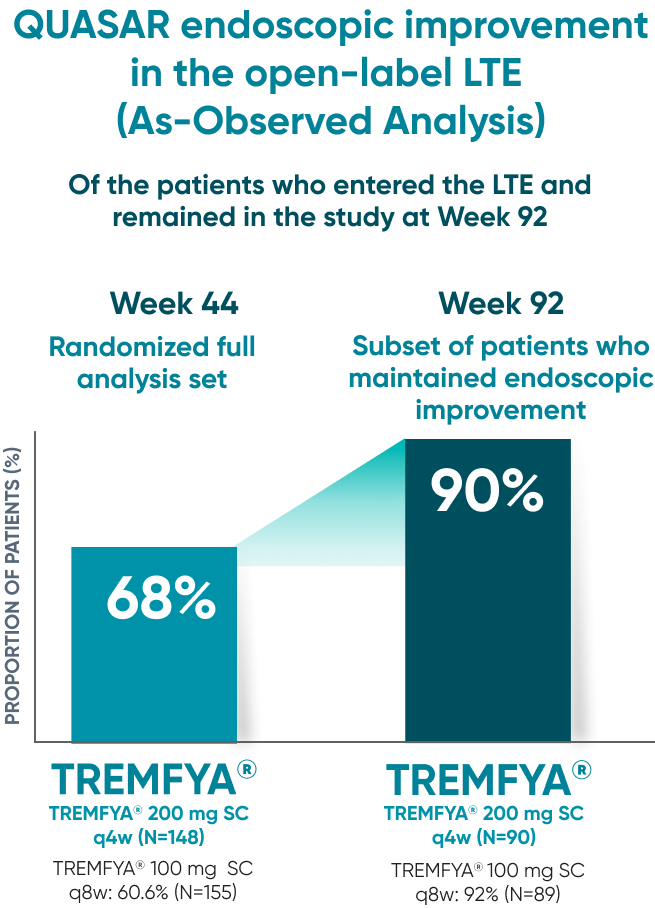
90% of patients on TREMFYA® who showed endoscopic improvement‡ at 1 year maintained endoscopic improvement at 2 years
As-observed (AO) analysis: All observed data were analyzed exactly as collected, with missing data excluded from calculations for that visit, and regardless of whether patients experienced events during the study that could have affected the outcome measurement (such as discontinuing study intervention or having an ostomy or colectomy).
DATA LIMITATION: Maintenance of endoscopic improvement at Week 92 during the LTE was prespecified but not controlled. Patients who dropped out of the LTE or who had missing data were not included in this as-observed analysis, which may increase the percentages of responders. No statistical significance can be made.
Use the lowest effective recommended dosage to maintain therapeutic response.
*Week 44 of the maintenance study was defined as 1 year. Week 92 of the LTE was defined as 2 years.
†LTE randomized full analysis set. Data are shown for participants who were in clinical response to TREMFYA® IV induction dosing and were randomized on entry into the maintenance study and did not experience a dose adjustment (including a sham dose adjustment) from Week 8 through Week 32.
‡Endoscopic improvement was defined as endoscopy subscore of 0 or 1 with no friability as assessed by a central reader. After Week 44 of maintenance study, only the local read, without friability, was available.
IV=intravenous; LTE=long-term extension; q4w=every 4 weeks; q8w=every 8 weeks; SC=subcutaneous.




